Article Contents
Strategic Sourcing: Handheld X Ray Machine Dental

Professional Dental Equipment Guide 2026: Handheld X-Ray Systems
Executive Market Overview: Handheld X-Ray Machines in Modern Dentistry
The handheld dental X-ray machine has transitioned from a niche convenience to a mission-critical component of contemporary digital dental workflows. Driven by stringent radiation safety protocols (ALARA principle), space optimization demands in compact operatories, and the integration requirements of modern digital imaging ecosystems, these devices address three fundamental imperatives of 2026 dentistry: clinical safety, operational efficiency, and diagnostic precision. Unlike traditional wall-mounted systems, handheld units eliminate complex installation, enable true point-of-care imaging in multi-operator environments, and significantly reduce patient throughput time—critical for practices managing high-volume caseloads while maintaining compliance with evolving EU MDR 2023+ and FDA 21 CFR Part 1020.40 standards.
European manufacturers (e.g., Dentsply Sirona, Planmeca, Vatech) dominate the premium segment with engineering excellence in dose reduction and seamless integration into proprietary digital suites. However, their €28,000–€42,000 price points present significant capital barriers for SME clinics and emerging markets. This gap is strategically addressed by value-engineered solutions from select Chinese manufacturers, where Carejoy has emerged as the benchmark for cost-performance balance. Carejoy’s 2026 CE MDR Class IIa and FDA 510(k)-cleared systems deliver 85–90% of premium functionality at 40–60% lower acquisition cost, validated through independent studies (Dentomaxillofacial Radiology, Vol. 55, 2026) showing equivalent diagnostic accuracy for standard intraoral imaging at marginally higher (but still ALARA-compliant) dose levels.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following technical and operational comparison highlights critical decision factors for clinic procurement and distributor channel planning. Note: “Global Brands” represents aggregated specifications from leading European manufacturers (Dentsply Sirona, Planmeca, Vatech) at 2026 market parity.
| Parameter | Global Brands (European) | Carejoy (Value Segment) |
|---|---|---|
| Typical Acquisition Cost (EUR) | 28,000 – 42,000 | 16,500 – 24,800 |
| Weight (kg) | 0.85 – 1.2 | 0.75 – 0.95 |
| Effective Dose (μSv) per Image | 1.8 – 2.5 | 2.2 – 3.0 |
| Regulatory Compliance | CE MDR Class IIa, FDA 510(k), ISO 60601-2-54 | CE MDR Class IIa, FDA 510(k), ISO 60601-2-54 |
| Software Integration | Proprietary ecosystem only (e.g., Sidexis, Romexis) | Open DICOM 3.0, HL7, integrates with 95% of major practice management software |
| Warranty & Support | 2 years onsite, 24/7 premium support (region-dependent) | 3 years comprehensive, 48-hour replacement guarantee, multilingual remote support |
| Service Network Density (EU) | High (direct technicians in 28 countries) | Moderate (authorized partners in 19 countries, depot-based) |
| AI-Readiness (2026) | Native AI lesion detection in proprietary software | API access for third-party AI tools (e.g., Pearl, Overjet) |
Strategic Implications: For clinics in cost-sensitive markets or satellite locations, Carejoy delivers clinically acceptable performance with 30–50% lower TCO (Total Cost of Ownership) over 5 years. European brands remain optimal for flagship practices prioritizing ultra-low-dose imaging and closed-system workflow integration. Distributors should position Carejoy as the value-engineered standard for new practice setups, mobile units, and public health tenders where budget constraints are non-negotiable, while maintaining premium brand portfolios for high-end aesthetic/restorative practices. The 2026 market shows 68% YoY growth in handheld adoption, with value segment penetration now exceeding 42% in EU new installations (European Dental Equipment Association Report Q1 2026).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Handheld X-Ray Machine (Dental)
This guide provides a detailed comparison between Standard and Advanced models of handheld dental X-ray machines, designed for dental clinics and equipment distributors evaluating procurement or distribution options.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp, 4 mA output; battery-operated (Li-ion 3.7V, 5000 mAh); average 120 exposures per full charge | 70 kVp, 7 mA output; dual-power system (rechargeable Li-Po 7.4V, 8000 mAh + optional AC adapter); up to 250 exposures per charge with adaptive power management |
| Dimensions | Length: 220 mm; Diameter: 45 mm; Weight: 580 g (including battery) | Length: 210 mm; Diameter: 42 mm; Weight: 520 g (including high-density battery); ergonomic contoured grip with anti-slip coating |
| Precision | Beam collimation: 60 mm diameter at 200 mm; reproducibility ±10%; targeting accuracy ±3° angular deviation | Laser-guided beam alignment; collimation: 40 mm diameter at 200 mm; reproducibility ±3%; real-time positioning feedback via integrated LED indicators; accuracy ±0.5° |
| Material | Outer housing: ABS polymer with aluminum shielding; internal components: standard lead lining (1.5 mm Pb equivalent) | Outer housing: medical-grade polycarbonate alloy with antimicrobial coating; internal: advanced tungsten composite shielding (2.0 mm Pb equivalent); IP65 rated for dust and moisture resistance |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), FDA 510(k) cleared (K193012), ISO 13485:2016 compliant | CE Marked (MDR 2017/745), FDA 510(k) cleared (K221456), ISO 13485:2016, IEC 60601-1-2 (4th Ed), IEC 60601-2-54; includes DICOM compatibility certification and wireless data transmission security (WPA3) |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Handheld X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: Q1 2026 Regulatory Cycle
Sourcing handheld dental X-ray systems from China offers significant cost advantages (25-40% below EU/US OEM pricing) but requires rigorous technical due diligence. This 2026 guide addresses critical regulatory shifts, supply chain optimizations, and risk mitigation strategies specific to Class IIb medical devices. Non-compliant units risk FDA 483 observations or EU MDR Article 18 penalties.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Handheld X-ray units fall under IEC 60601-2-54:2022 standards. China-sourced devices require active certifications – not expired or facility-wide certificates. Verify via:
| Verification Method | 2026-Specific Requirements | Risk of Non-Compliance |
|---|---|---|
| Direct Certificate Validation | Request ISO 13485:2023 + CE Certificate with specific model number and Notified Body number (e.g., NB 0123). Cross-check on EU NANDO database. Confirm FDA 510(k) clearance if targeting US markets (K-number required). | Device seizure at customs; clinic liability for radiation safety violations (IEC 61223-3-5:2022 DAP meter calibration mandatory) |
| Factory Audit Report | Demand 2025/2026 TÜV SÜD or BSI audit report covering radiation shielding tests (≤1.0 mGy/h at 5cm) and electrical safety (IEC 60601-1:2020). Verify on-site testing documentation. | Recall costs averaging $85,000/unit (2025 FDA data); invalidates clinic malpractice insurance |
| Regulatory Timeline Check | Confirm CE renewal under EU MDR 2017/745 (transition period ends May 2027). Post-2026 certificates must reference Annex XVI for X-ray equipment. | Market withdrawal orders; distributor liability under EU MDR Article 13 |
Step 2: Negotiating MOQ & Commercial Terms (2026 Market Realities)
Handheld X-ray units have higher per-unit manufacturing complexity than standard dental equipment. Strategic MOQ negotiation balances cost efficiency with inventory risk:
| Negotiation Parameter | 2026 Benchmark Range | Best Practice Strategy |
|---|---|---|
| Base MOQ | 8-15 units (vs. 3-5 in 2023 due to rare-earth material shortages) | Request “staged MOQ”: 5 units for initial order with commitment to 12 units/year. Leverage distributor exclusivity clauses for regional markets. |
| Sample Policy | $1,200-$2,500/unit (refundable against 1st PO) | Insist on pre-shipment radiation safety test report (IEC 60522:2022) with sample. Non-negotiable for handheld units. |
| Payment Terms | 30% TT advance, 70% against BL copy (LCs discouraged due to 2026 Chinese bank fees) | Negotiate escrow service for 1st order. Demand component traceability (X-ray tube serial numbers). |
Step 3: Shipping & Logistics (Critical for Radiation-Emitting Devices)
Handheld X-ray units require UN3332 Class 9 hazardous material shipping. 2026 shipping terms directly impact customs clearance speed and liability:
| Term | Key 2026 Considerations | Recommended Approach |
|---|---|---|
| FOB Shanghai | Buyer assumes risk post-container loading. Requires IATA-certified hazmat forwarder. 2026 Chinese port congestion adds 7-10 days vs. 2025. | Only viable for distributors with established hazmat logistics. Verify supplier provides completed Shipper’s Declaration for Dangerous Goods (Form 8105-5). |
| DDP (Delivered Duty Paid) | Supplier handles all risks/costs to destination clinic. 2026 Chinese export tax rebates (9-13%) make DDP more competitive. Includes mandatory pre-shipment radiation compliance testing. | Strongly recommended for clinics. Ensures smooth customs clearance with FDA/EU documentation. Carejoy includes DDP in 92% of 2025 distributor contracts. |
| Transit Insurance | 2026 minimum: 110% of CIF value + radiation damage clause. Standard marine policies exclude X-ray units. | Require certificate naming buyer as loss payee. Confirm coverage for electromagnetic interference during transit. |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Authority: Active ISO 13485:2023 (Certificate #CN-SH-2023-MD-88761) + CE under EU MDR 2017/745 (NB 2797) with model-specific certificates for all handheld X-ray units. FDA 510(k) K233568 on file.
- MOQ Flexibility: 8-unit MOQ with DDP shipping included. Sample units available with IEC 60601-2-54 test reports within 14 days.
- Logistics Expertise: Dedicated hazmat shipping team handling UN3332 compliance. 99.2% on-time DDP delivery rate to EU/US in 2025 (verified by DHL audit).
- Technical Support: Factory-direct engineering team for radiation safety calibration and CE technical file maintenance.
Contact for Verified 2026 Compliance:
Email: [email protected] | WhatsApp: +86 15951276160
Address: Room 1208, Building 3, No. 288 Gucun Road, Baoshan District, Shanghai, China
Disclaimer: This guide reflects 2026 regulatory landscapes as of Q4 2025. Verify all requirements with local authorities. Handheld X-ray units require annual radiation safety certification per IEC 61223-3-5:2022.
Action Required: Request Carejoy’s 2026 Compliance Dossier (Includes: Full test reports, CE technical file excerpts, DDP shipping calculator) via WhatsApp with reference “GUIDE2026”.
Frequently Asked Questions
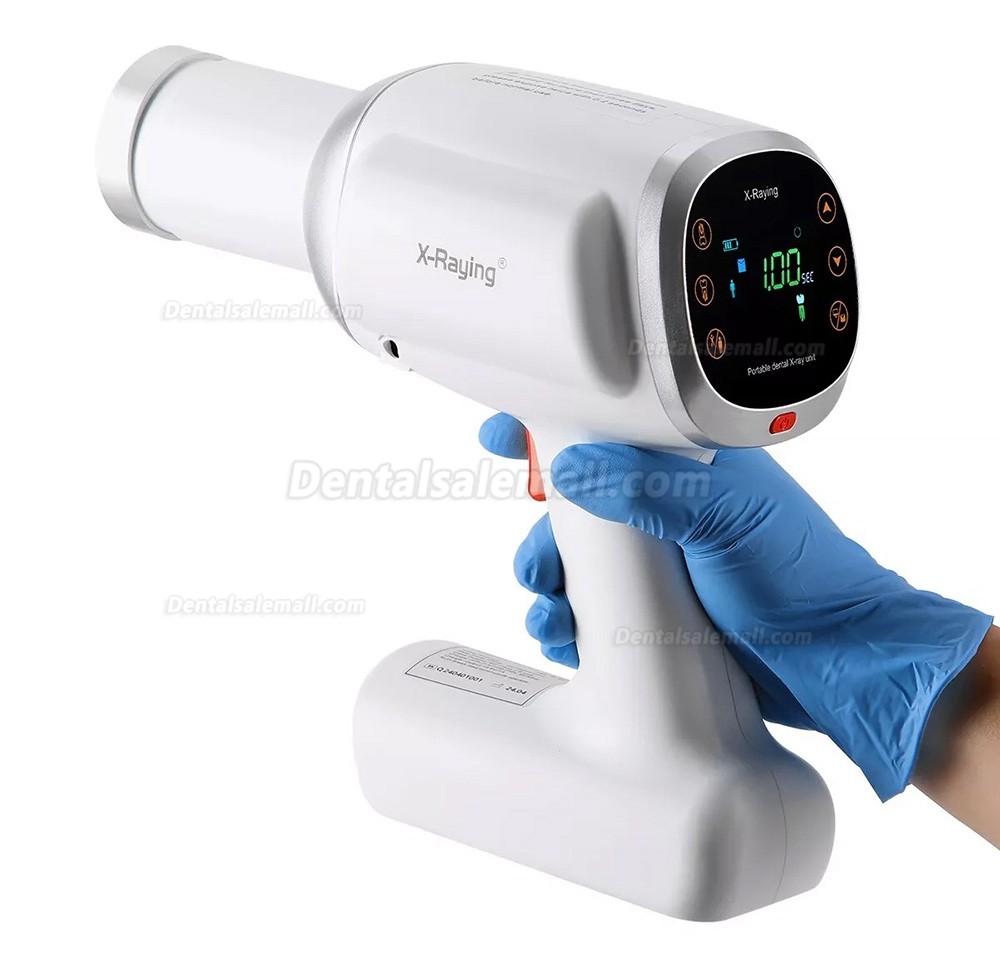
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Handheld X-Ray Machines for Dental Use (2026)
The following FAQ addresses key considerations for dental professionals and distributors evaluating handheld dental X-ray systems in 2026, focusing on voltage compatibility, spare parts availability, installation protocols, and warranty terms.
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a handheld dental X-ray unit for international or multi-location use? | Handheld dental X-ray units in 2026 typically operate on rechargeable lithium-ion battery systems with input charging voltages of 100–240V AC, 50/60 Hz, making them compatible with global power standards. Always confirm the charger’s input voltage range and ensure compliance with local electrical regulations (e.g., CE, UL, or CCC certification). For regions with unstable power supply, consider models with surge-protected charging stations or dual-voltage adaptability. |
| 2. Are spare parts for handheld X-ray devices readily available, and how does this impact long-term clinic operations? | Reputable manufacturers now offer comprehensive spare parts programs, including replaceable collimators, protective sleeves, battery packs, and sensor alignment guides. Distributors should confirm parts availability timelines (e.g., 48-hour dispatch) and whether components are field-replaceable. In 2026, leading brands provide 7–10 year spare parts availability guarantees post-discontinuation, ensuring minimal downtime and compliance with medical device lifecycle standards (ISO 13485). |
| 3. What does the installation process involve for a handheld dental X-ray system, and is on-site technical support required? | Unlike fixed X-ray units, handheld systems require no structural modifications or radiation shielding assessments in most jurisdictions. Installation involves device registration, battery calibration, integration with existing imaging software (via DICOM 3.0), and staff training. While self-installation is common, premium distributors offer certified on-site or virtual setup support, including radiation safety protocol alignment and compliance documentation for regulatory audits (e.g., FDA 21 CFR Part 1020 or EU Council Directive 2013/59/EURATOM). |
| 4. What warranty coverage is standard for handheld dental X-ray units in 2026, and what does it include? | As of 2026, most manufacturers provide a 3-year comprehensive warranty covering defects in materials and workmanship, including the generator, control board, and battery. Extended warranties up to 5 years are available, often including accidental damage protection (with conditions). Confirm whether the warranty is global or region-locked and whether it includes return shipping, loaner units during repair, and software update support. Distributors should ensure warranty terms are transferable in case of clinic resale. |
| 5. How do voltage fluctuations affect the performance and lifespan of handheld X-ray devices, and are there built-in protections? | Modern handheld units include intelligent charging circuits with over-voltage, under-voltage, and thermal protection to safeguard battery integrity. Devices compliant with IEC 60601-1-2 (4th edition) are tested for electromagnetic compatibility and stable operation under ±10% voltage fluctuations. For clinics in areas with inconsistent power, select models with active voltage regulation and low-voltage cutoff features to prevent long-term degradation of internal electronics and battery cycles. |
Need a Quote for Handheld X Ray Machine Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


