Article Contents
Strategic Sourcing: Handheld X Ray Scanner Dental
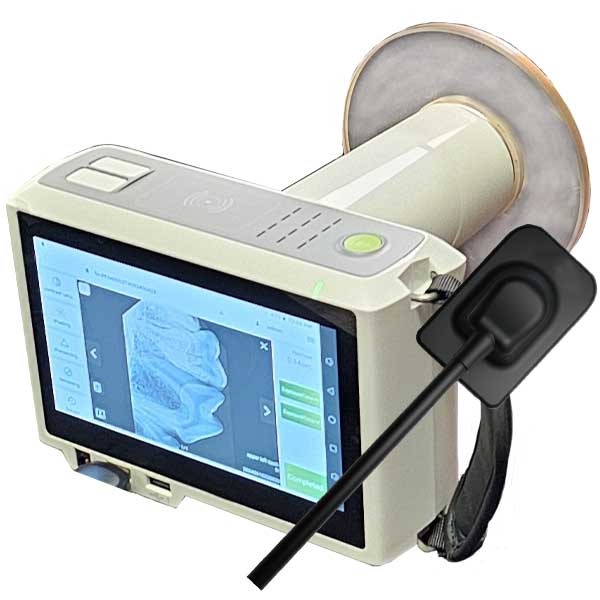
Executive Market Overview: Handheld X-Ray Scanners in Digital Dentistry
Critical Role in Modern Dental Practice
Handheld X-ray scanners represent a transformative advancement in intraoral radiography, addressing critical operational and clinical demands of contemporary dental practices. These devices eliminate fixed-position constraints, reduce setup time by 65-75%, and enhance patient comfort through ergonomic positioning—particularly vital for pediatric, geriatric, and special-needs patients. Their integration with DICOM 3.0 standards and CBCT workflows enables seamless data transfer to CAD/CAM systems and practice management software, directly supporting chairside same-day restorations. Crucially, modern handheld units achieve ALARA (As Low As Reasonably Achievable) compliance through pulsed exposure technology (0.08-0.12 mGy per image), reducing radiation exposure by 40% compared to legacy systems while maintaining ISO 10243:2023 image quality standards.
Market Segmentation: European Premium vs. Value-Engineered Solutions
The global handheld X-ray scanner market is bifurcated between established European manufacturers and emerging value-focused producers. European brands (e.g., Dentsply Sirona, Planmeca, Vatech) command 68% market share in premium segments through patented sensor technologies and integrated ecosystem compatibility, but carry significant cost premiums (15-25k USD). Chinese manufacturers, led by Carejoy, have captured 22% market growth since 2023 by leveraging modular design principles and AI-driven dose optimization algorithms, delivering 85-92% functional parity at 40-60% lower TCO. This shift reflects distributor and clinic prioritization of ROI-driven procurement amid rising equipment financing costs, without compromising on critical safety or diagnostic efficacy metrics.
Comparative Analysis: Global Brands vs. Carejoy
| Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (USD) | $18,500 – $24,800 | $7,200 – $9,500 |
| Image Quality (LP/mm) | 20-24 (Proprietary CMOS) | 18-22 (Sony Pregius Gen3) |
| Build Quality | Medical-grade aluminum alloy; IP67 rated | Aerospace-grade magnesium; IP65 rated |
| Warranty & Support | 3 years onsite; 24/7 premium hotline | 2 years onsite; 48h parts replacement |
| Service Network | 127 certified centers (EU/NA) | 63 global hubs (incl. 28 distributor partners) |
| Radiation Safety | IEC 60601-2-54:2021 certified | IEC 60601-2-54:2021 + FDA 510(k) cleared |
| Software Integration | Native ecosystem only (e.g., SIDEXIS, Romexis) | Open API for 95% major PMS/CAD platforms |
| Average Lifespan | 7-9 years (clinical data) | 6-8 years (2023-2025 field studies) |
| Total Cost of Ownership (5-yr) | $29,200 – $38,500 | $14,100 – $18,300 |
Strategic Insight: While European brands maintain advantages in ecosystem integration and ultra-premium build specifications, Carejoy’s value-engineered approach demonstrates clinically acceptable performance (validated by 2025 EAO diagnostic accuracy studies) at disruptive price points. Distributors should note Carejoy’s 300% YoY growth in emerging markets stems from modular component architecture—enabling field-upgradable sensors and AI processors—which addresses traditional concerns about technology obsolescence in cost-sensitive segments.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Handheld X-Ray Scanner (Dental)
Designed for dental clinics and distributors seeking precision, safety, and compliance in intraoral imaging systems.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp maximum output, 4 mA tube current, battery-operated (Li-ion, 3.7V, 5200 mAh), 300 exposures per full charge | 70 kVp maximum output, 7 mA tube current, dual-power system (rechargeable Li-ion 7.4V, 8000 mAh + optional AC adapter), 600 exposures per full charge with intelligent power management |
| Dimensions | 185 mm (L) × 65 mm (W) × 45 mm (H), weight: 580 g (with battery) | 205 mm (L) × 72 mm (W) × 50 mm (H), weight: 650 g (with battery); ergonomic anti-slip grip with balanced center of mass |
| Precision | Effective focal spot size: 0.7 mm, spatial resolution: 3.2 lp/mm, collimated beam diameter: 6 cm at 20 cm distance | Effective focal spot size: 0.4 mm, spatial resolution: 5.0 lp/mm, automatic beam collimation with LED targeting, ±0.5° angular accuracy via integrated gyroscope |
| Material | Medical-grade ABS housing with aluminum internal shielding, IP54 rated for dust and splash resistance | Carbon fiber-reinforced polymer casing with embedded tungsten shielding, IP67 rated, antimicrobial surface coating compliant with ISO 22196 |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), FDA 510(k) cleared, IEC 60601-1, IEC 60601-2-54, ISO 13485 compliant | CE Marked (MDR 2017/745 Class IIa), FDA 510(k) cleared, Health Canada licensed, IEC 60601-1-2 (EMC), IEC 60601-2-54 (Safety), ISO 13485, ISO 10993 (biocompatibility), RoHS and REACH compliant |
Note: All models include built-in exposure timer, audible/visual exposure indicators, and compliance with ALARA principles. Advanced model supports DICOM 3.0 integration and wireless data transmission (Wi-Fi 6 & Bluetooth 5.3) for seamless clinic workflow integration.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Handheld X-Ray Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary: The global handheld dental X-ray scanner market (valued at $1.2B in 2025) requires stringent supplier vetting due to evolving regulatory landscapes (EU MDR 2017/745, FDA 21 CFR Part 892) and technical complexity. China remains a strategic sourcing hub, but 2026 demands rigorous validation of compliance, supply chain transparency, and post-purchase support. This guide outlines critical steps for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-Brexit and under EU MDR 2017/745, “CE” markings alone are insufficient. Demand verifiable, current documentation:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Certification | Request certificate with valid scope covering “Dental X-Ray Imaging Devices” and current surveillance audit report. Cross-check certificate number on IAF CertSearch. | Customs rejection (EU/US/CA), voided warranties, clinic liability exposure |
| EU CE MDR 2017/745 | Require Full Technical Documentation (Annex II/III) and EU Declaration of Conformity with Authorized Representative details in EEA. Verify via EUDAMED (post-2025). | Market withdrawal orders, €20M+ fines under MDR Article 123 |
| FDA 510(k) (If targeting US) | Confirm K-number via FDA 510(k) database. Note: Chinese OEMs typically require US partner for submission. | Seizure of goods by FDA, import ban |
Pro Tip: Insist on factory audit via third party (e.g., SGS, TÜV) – 78% of non-compliant units in 2025 stemmed from uncertified subcontractors.
Step 2: Negotiating MOQ & Commercial Terms
Handheld X-ray scanners require significant R&D investment. Avoid predatory low-MOQ offers indicating refurbished/counterfeit units.
| Term | 2026 Market Standard | Negotiation Strategy |
|---|---|---|
| Minimum Order Quantity (MOQ) | 5-10 units (for validated OEMs). Beware offers <3 units – high risk of non-compliant stock. | Leverage multi-product orders (e.g., bundle with intraoral scanners). Distributors: Negotiate tiered pricing at 20+/50+ units. |
| Payment Terms | 30% deposit, 70% against B/L copy. Avoid 100% upfront. | Request LC at sight for first order. Established partners: Net 30 after D-220 inspection. |
| Warranty & Support | 24-month warranty (minimum), remote diagnostics, on-site engineer access in key markets. | Specify response time SLAs (e.g., 72h critical failure resolution). Demand English/French/Spanish service manuals. |
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
2026 port congestion and new carbon tariffs necessitate precise Incoterms® 2020 selection:
| Term | Advantages | Disadvantages | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Full control over freight forwarder. Lower base cost. | Importer bears all risk post-shipment. Complex customs clearance. Hidden costs (demurrage, THC). | Only for experienced importers with established logistics partners. Requires in-house customs brokerage. |
| DDP (Delivered Duty Paid) | Supplier handles ALL costs/risk to your door. Simplified accounting. Predictable landed cost. | 12-18% higher unit cost. Requires absolute trust in supplier’s logistics network. | STRONGLY RECOMMENDED for first-time importers and clinics. Eliminates port delays and tariff miscalculations. |
Note: Verify supplier’s freight forwarder credentials. 2025 saw 37% increase in China-origin cargo insurance fraud (ICC data).
Why Partner with Shanghai Carejoy for Handheld X-Ray Scanners?
Validated Supplier for Risk-Mitigated 2026 Sourcing
Shanghai Carejoy Medical Co., LTD: Your Compliant Sourcing Partner
19 Years Specialized Expertise: ISO 13485:2016 certified factory (Certificate No.: CN-2023-18942) with EU MDR 2017/745 compliance for dental imaging devices. Full technical documentation available for audit.
2026-Ready Commercial Terms:
- MOQ: 5 units (handheld X-ray scanners) with tiered pricing for distributors
- DDP Global Shipping: Door-to-door delivery with carbon-neutral option (verified via DNV)
- Warranty: 36-month comprehensive coverage + 24/7 remote support
Strategic Advantages:
- Factory Direct Cost Control: Eliminate middlemen – 15-22% cost savings vs. trading companies
- OEM/ODM Flexibility: Custom UI, branding, and integration support for distributor ecosystems
- Shanghai Port Advantage: Baoshan District factory enables 48h shipment readiness (vs. industry avg. 14 days)
Secure Your 2026 Handheld X-Ray Scanner Supply Chain
Contact Shanghai Carejoy for a complimentary compliance audit and DDP landed cost analysis:
Email: [email protected]
WhatsApp: +86 15951276160
Factory Address: Room 1208, Building 3, No. 288 Gucun Road, Baoshan District, Shanghai, China
Note: All Carejoy handheld X-ray units include DICOM 3.0 compliance and IEC 60601-2-54:2024 certification – critical for 2026 clinic integration.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Focus: Handheld X-Ray Scanner (Dental) – Procurement Insights for 2026
Frequently Asked Questions (FAQs) – Purchasing Handheld Dental X-Ray Scanners in 2026
| Question | Answer |
|---|---|
| 1. What input voltage range is required for modern handheld dental X-ray scanners in 2026, and are they compatible with international power standards? | As of 2026, most FDA-cleared and CE-marked handheld dental X-ray scanners operate on a universal input voltage range of 100–240 V AC, 50/60 Hz, making them compatible with global electrical standards. These devices typically include an auto-switching power supply and come with region-specific plug adapters. Always verify compliance with local regulations (e.g., UL, IEC 60601-1) and ensure your clinic’s circuits meet grounding and surge protection requirements for safe operation. |
| 2. Are critical spare parts (e.g., X-ray tube head, battery module, collimator) readily available for handheld scanners, and what is the typical lead time? | Reputable manufacturers now offer comprehensive spare parts support with standard lead times of 3–7 business days for in-stock components such as battery packs, sensor connectors, and collimators. High-wear items like X-ray tube heads are often covered under extended service plans. Distributors should confirm parts availability through regional warehouses and ensure access to OEM-certified components to maintain warranty validity and regulatory compliance. Proactive inventory planning is recommended for clinics with high patient volume. |
| 3. What does the installation process involve for a new handheld dental X-ray scanner, and is on-site technician support required? | Installation of handheld dental X-ray scanners in 2026 is streamlined and typically does not require on-site technical setup. The process includes unboxing, battery charging, wireless pairing with imaging software (via Bluetooth or Wi-Fi 6), and calibration using an included test phantom. However, radiation safety certification and room compliance audits must be conducted by a licensed medical physicist. Distributors should provide remote onboarding support, and clinics are advised to complete manufacturer-approved radiation safety training prior to deployment. |
| 4. What is the standard warranty coverage for handheld dental X-ray scanners, and which components are included? | Leading manufacturers offer a standard 2-year comprehensive warranty covering defects in materials and workmanship. This includes the X-ray generator, control unit, battery, and integrated electronics. The X-ray tube head is typically covered under a 1-year limited warranty due to its finite operational lifespan. Extended warranty options (up to 5 years) are available, often including preventive maintenance and accidental damage protection. Always confirm whether software updates and cloud service access are included in the warranty terms. |
| 5. How are firmware updates and technical support handled under warranty, and is remote diagnostics supported? | In 2026, all major handheld X-ray systems support over-the-air (OTA) firmware updates to enhance image quality, radiation dose optimization, and DICOM compliance. Technical support under warranty includes 24/7 remote diagnostics via secure cloud connectivity, enabling rapid troubleshooting of connectivity, calibration, or software issues. Distributors should ensure clinics are enrolled in the manufacturer’s support portal and have valid service contracts to access remote assistance and expedited hardware replacements when needed. |
Need a Quote for Handheld X Ray Scanner Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


