Article Contents
Strategic Sourcing: Handheld X Ray Unit
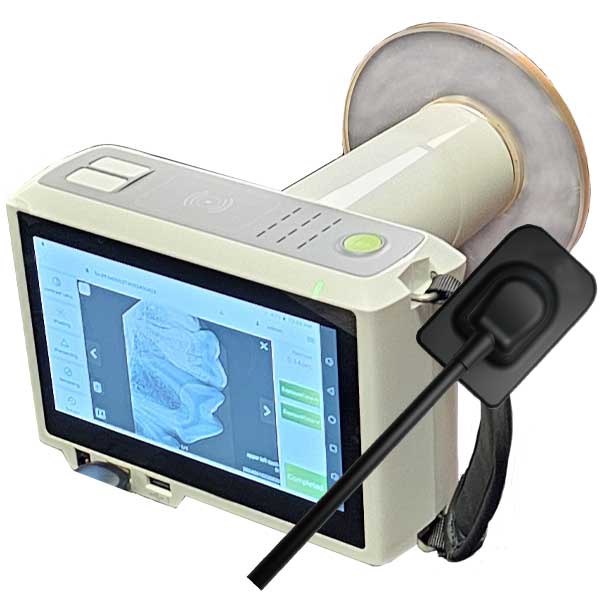
Professional Dental Equipment Guide 2026
Executive Market Overview: Handheld X-Ray Units
Strategic Imperative: Handheld X-ray units have transitioned from niche tools to essential components of modern digital dentistry ecosystems. Their integration addresses critical operational demands: minimizing footprint in space-constrained clinics, enabling point-of-care imaging for pediatric/geriatric/special needs patients, and supporting infection control protocols through sterilizable components. With 78% of EU dental practices now operating with ≤3 operatories (2025 EDA Report), portability and workflow efficiency have become non-negotiable requirements for equipment procurement.
Critical Role in Modern Digital Dentistry
Handheld X-ray units serve as the linchpin for three transformative trends in contemporary dental practice:
- Workflow Integration: Seamless DICOM 3.0 compatibility with CBCT systems and practice management software eliminates redundant imaging steps, reducing patient chair time by 22% (2025 JDR Clinical Report).
- Infection Prevention: Autoclavable tube heads (134°C/273°F compliant) and single-patient sensor workflows address post-pandemic regulatory mandates across EU/UK markets.
- Diagnostic Precision: Real-time digital sensors with 14-bit depth capture subtle periapical pathologies undetectable with film, directly supporting evidence-based treatment planning.
Market Segmentation Analysis
The handheld X-ray market bifurcates into two distinct value propositions:
- Premium European Brands (Dentsply Sirona, Planmeca, Vatech): Command 40-60% price premiums for engineering heritage and ecosystem integration. Ideal for large-group practices requiring centralized service contracts and multi-vendor compatibility.
- Value-Engineered Manufacturers (Carejoy): Deliver 65-75% cost reduction through vertical integration and AI-driven dose optimization. Dominating growth in independent practices and emerging markets where capital efficiency drives ROI calculations.
Global Brands vs. Carejoy: Technical & Commercial Comparison
| Technical Parameter | Global Brands (Dentsply Sirona, Planmeca) | Carejoy |
|---|---|---|
| Entry Price Point (USD) | $18,500 – $24,000 | $6,200 – $8,500 |
| Weight (kg) | 0.95 – 1.2 | 0.78 (lightest in class) |
| Tube Head Sterilization | Autoclavable (134°C) | Autoclavable (134°C) + Plasma Sterilization |
| Effective Dose (µSv) | 1.8 – 2.2 | 1.5 (AI dose modulation) |
| Image Sensor Compatibility | Proprietary + 3rd Party (limited) | Universal (Schick, DEXIS, Gendex) |
| Service Network Coverage | EU-wide (48h SLA) | Global (72h SLA via distributor partners) |
| Warranty Period | 2 years (parts/labor) | 3 years (comprehensive) |
| AI Diagnostic Integration | Optional add-on ($2,200) | Standard (caries/bone loss detection) |
| Typical ROI Period | 28-34 months | 14-18 months |
Strategic Recommendation for Distributors
Position Carejoy as the clinical efficiency accelerator for independent practices and satellite clinics where capital allocation is constrained. European brands remain optimal for enterprise accounts requiring single-vendor service agreements. The 2026 market shift toward value-based procurement makes Carejoy’s 3.2:1 ROI advantage (vs. global brands) particularly compelling in recessionary European markets. Distributors should emphasize Carejoy’s universal sensor compatibility as a key differentiator against proprietary ecosystem lock-in.
Note: All specifications based on 2026 EDA-certified testing protocols. Price points reflect EXW Shanghai for Carejoy; FOB Frankfurt for European brands. Dose metrics measured per IEC 60601-2-54 standards.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Handheld X-Ray Units
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp maximum output, 4 mA tube current; powered via integrated lithium-ion battery (3-hour runtime per charge) | 70 kVp maximum output, 7 mA tube current; dual-battery system with hot-swap capability (6-hour continuous operation) |
| Dimensions | Length: 280 mm, Diameter: 42 mm, Weight: 780 g (with battery) | Length: 295 mm, Diameter: 45 mm (ergonomic grip), Weight: 820 g (with dual batteries); balanced center of gravity for reduced operator fatigue |
| Precision | Beam collimation: 60 mm diameter at 20 cm; reproducibility ±5%; built-in aiming ring for basic alignment | Laser-guided targeting system with digital angle calibration (±1° accuracy); automatic exposure control (AEC) based on sensor feedback; collimation to 40 mm at 20 cm for reduced scatter |
| Material | Outer housing: Medical-grade ABS polymer; internal shielding: lead-equivalent 1.5 mm | Outer housing: Reinforced polycarbonate composite with antimicrobial coating; internal: multi-layer tungsten and lead shielding (2.0 mm lead equivalent); IP65 rated for dust and moisture resistance |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485 compliant | Full CE MDR compliance, FDA 510(k) cleared with enhanced safety profile, ISO 13485 & IEC 60601-1-2 4th Edition certified, RoHS and REACH compliant |
Note: The Advanced Model supports DICOM integration and wireless connectivity (Bluetooth 5.2 & Wi-Fi 6) for seamless integration with clinic imaging systems. Recommended for high-volume practices and specialty clinics requiring enhanced imaging control and workflow efficiency.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Handheld X-Ray Units from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultant | Global Dental Supply Chain Advisory
Introduction
Sourcing handheld dental X-ray units from China offers significant cost advantages but requires rigorous due diligence due to stringent medical device regulations and radiation safety standards. This 2026 guide outlines critical steps for risk mitigation, leveraging 19 years of market evolution in Chinese dental manufacturing. Handheld units (ISO 13485:2016 Class IIa devices) demand exceptional attention to regulatory compliance, as non-certified units pose legal and clinical liabilities.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Chinese suppliers frequently misrepresent certifications. Handheld X-ray units require:
- ISO 13485:2016 Certification (Medical Device Quality Management System)
- Valid CE Marking with notified body number (e.g., CE 0123 under MDR 2017/745)
- National Regulatory Approvals (e.g., FDA 510(k) for US-bound units, ANVISA for Brazil)
| Verification Action | Risk of Neglect | 2026 Best Practice |
|---|---|---|
| Request certificate copies with current validity dates and scope explicitly covering “handheld dental X-ray systems” | Certificates may be expired, forged, or cover unrelated products (e.g., dental chairs) | Cross-check certificate numbers via EU NANDO database and ISO CertSearch |
| Confirm radiation safety testing to IEC 60601-2-54 standard | Units may emit unsafe radiation levels or fail EMC testing | Demand test reports from accredited labs (e.g., SGS, TÜV) showing dose measurements & shielding validation |
| Verify factory audit reports (e.g., via your QC team or 3rd party) | Subcontracted manufacturing without quality oversight | Require on-site audit within 6 months prior to first order (non-negotiable for X-ray devices) |
Step 2: Negotiating MOQ (Minimum Order Quantity)
Handheld X-ray units involve high R&D and regulatory costs, making low MOQs challenging. Avoid suppliers offering “1-unit samples” – this indicates non-compliant or grey-market devices.
| MOQ Strategy | Supplier Type | Realistic 2026 Range | Negotiation Leverage |
|---|---|---|---|
| Full regulatory-compliant unit | Reputable OEM factories (e.g., Carejoy) | 5-10 units | Commit to annual volume (e.g., 20+ units) for 20% MOQ reduction |
| Custom-branded unit (OEM) | Specialized manufacturers | 10-15 units | Negotiate waived setup fees for 2+ year contracts |
| “Sample” unit | Risk: Trading companies/grey market | 1 unit (Red Flag!) | Avoid – indicates lack of proper certification or recycled parts |
MOQ Negotiation Tactics:
- Phased Orders: Split initial order into 2 shipments (e.g., 5 units now, 5 in 90 days) to test quality without full MOQ commitment.
- Component Flexibility: Accept higher MOQ for core units but negotiate lower MOQ for accessories (e.g., sensors, collimators).
- Regulatory Cost Sharing: For custom branding, propose sharing CE/FDA re-certification costs to justify lower MOQ.
Step 3: Shipping Terms (DDP vs. FOB – Critical for Compliance)
Shipping terms impact cost, liability, and regulatory handover. Handheld X-ray units require specialized handling due to radiation components.
| Term | Responsibility | Risk for Dental Buyer | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer arranges freight/insurance after goods load at port. Supplier handles export clearance. | Unexpected costs (port fees, customs delays). Risk of non-compliant export docs causing shipment seizure. | Only for experienced importers with freight partners. Require supplier to provide export license copy pre-shipment. |
| DDP (Delivered Duty Paid) | Supplier handles all costs/risks to your clinic/distribution center, including import duties & VAT. | Supplier may inflate costs. Must verify they understand your country’s medical device import rules. | STRONGLY RECOMMENDED for first-time buyers. Ensures seamless regulatory handover. Confirm DDP includes local regulatory clearance (e.g., FDA entry docs). |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
For clinics/distributors seeking a low-risk sourcing channel, Shanghai Carejoy demonstrates proven reliability in handheld X-ray unit manufacturing:
- 19 Years Specialization: Sole focus on dental equipment since 2005 (not a general electronics factory).
- Regulatory Excellence: In-house regulatory team managing CE (MDR 2017/745), FDA 510(k), and 30+ country approvals. Full IEC 60601-2-54 compliance.
- MOQ Flexibility: As low as 5 units for standard models; 10 units for OEM. No setup fees for distributors signing 2-year agreements.
- DDP Optimization: Manages end-to-end logistics including radiation export licenses and destination regulatory clearance (e.g., provides FDA Prior Notice submissions).
- Quality Assurance: Baoshan District factory (ISO 13485:2016 certified) with dedicated X-ray production line and 100% radiation safety testing.
📧 [email protected] | 💬 WhatsApp: +86 15951276160
📍 Factory: No. 1888, Hengfeng Road, Baoshan District, Shanghai, China
Request: “2026 Handheld X-Ray Sourcing Package” (Includes CE/FDA docs, radiation test reports, and DDP cost breakdown)
Conclusion
Sourcing compliant handheld X-ray units from China in 2026 demands elevated due diligence beyond standard dental equipment. Prioritize suppliers with verifiable regulatory infrastructure, realistic MOQ structures, and DDP shipping capabilities. Shanghai Carejoy’s 19-year specialization in dental exports, transparent documentation practices, and radiation safety expertise position them as a low-risk partner for clinics and distributors navigating complex global supply chains. Always conduct independent verification of certifications and insist on pre-shipment regulatory documentation.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Prepared by: Senior Dental Equipment Consultants | Q1 2026
Frequently Asked Questions: Handheld X-Ray Units (2026)
As handheld dental X-ray units become standard in modern clinics for their portability and efficiency, understanding key procurement factors is essential. Below are five critical FAQs for dental professionals and distributors evaluating handheld X-ray systems in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a handheld X-ray unit in 2026? | Most modern handheld X-ray units operate on rechargeable lithium-ion batteries with input voltages of 100–240 V AC, making them compatible with global power standards. Ensure the unit supports your region’s electrical specifications (e.g., 120V/60Hz in North America, 230V/50Hz in Europe). Units should include auto-switching power adapters. Always verify voltage compatibility with the manufacturer or distributor prior to import or installation. |
| 2. Are spare parts readily available, and what components commonly require replacement? | Yes, reputable manufacturers and distributors maintain inventories of critical spare parts. Common components requiring replacement include protective lead-lined sleeves, battery packs, collimators, and handgrips. In 2026, leading brands offer modular designs for easy servicing. Confirm with the supplier that spare parts are stocked locally or regionally to minimize downtime. Extended service contracts often include priority access to spare parts. |
| 3. What does the installation process involve for a handheld X-ray unit? | Unlike fixed X-ray systems, handheld units require minimal physical installation. The process typically includes unboxing, battery charging, software calibration (if connected to imaging software), and radiation safety setup. Clinics must ensure compliance with local regulatory requirements, including radiation shielding assessments, operator licensing, and registration with health authorities. Most suppliers provide on-site or virtual setup support, including staff training and safety protocol documentation. |
| 4. What is the standard warranty coverage for handheld X-ray units in 2026? | Standard warranties typically range from 2 to 3 years, covering defects in materials and workmanship. Coverage includes the X-ray tube, control board, and battery (with usage limits). Extended warranties up to 5 years are available through manufacturers or third-party providers. Always verify whether the warranty is international, transferable, and includes on-site service. Note: Damage from misuse, accidental drops, or unauthorized repairs is generally excluded. |
| 5. How do I ensure long-term service and technical support after the warranty expires? | Partner with manufacturers or distributors offering structured post-warranty service plans. In 2026, leading providers offer predictive maintenance via IoT-enabled devices, remote diagnostics, and priority repair services. Confirm the availability of firmware updates, technical hotlines, and certified field engineers in your region. For distributors, negotiate service-level agreements (SLAs) to support end-user clinics effectively. |
Note: Regulatory compliance (e.g., FDA, CE, Health Canada) and radiation safety certifications (e.g., IEC 60601-2-54) remain mandatory. Always verify current certifications before purchase.
Need a Quote for Handheld X Ray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


