Article Contents
Strategic Sourcing: Handheld Xray Unit
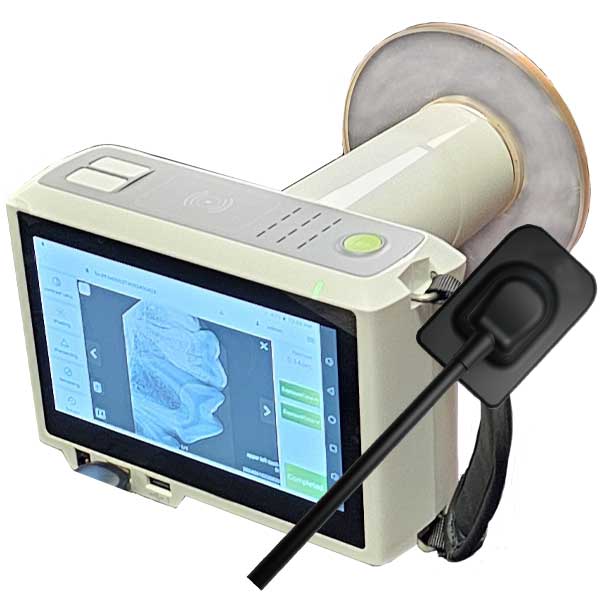
Professional Dental Equipment Guide 2026: Executive Market Overview
Handheld X-Ray Units – The Strategic Imperative for Modern Digital Dentistry
The global handheld X-ray unit market is projected to reach $1.2B by 2026 (CAGR 8.7%), driven by the irreversible shift toward digital dentistry, heightened infection control protocols, and demand for operational agility. These compact systems have evolved from niche tools to mission-critical infrastructure for forward-thinking practices, enabling seamless integration with CBCT, intraoral scanners, and cloud-based practice management software while addressing three fundamental modern clinical imperatives:
• Workflow Optimization: Eliminate room-bound limitations – capture periapicals, bitewings, and surgical site images in operatories, ORs, or nursing facilities without patient relocation.
• Infection Control Compliance: Fully autoclavable tubeheads (e.g., ISO 15223-1:2021 compliant) and wireless operation reduce cross-contamination vectors versus wall-mounted systems.
• Digital Ecosystem Integration: Native DICOM 3.0 output with AI-powered dose optimization (e.g., 0.5s exposure at 0.005mGy) ensures compatibility with AI diagnostics platforms and EHR systems.
As clinics prioritize capital efficiency without compromising diagnostic integrity, the procurement landscape bifurcates between premium European engineering and value-optimized Asian manufacturing. European leaders maintain dominance in high-acuity applications (e.g., endodontics, implantology), while Chinese innovators like Carejoy are rapidly closing the technology gap for routine diagnostics – a trend reshaping distributor channel strategies globally.
Strategic Procurement Analysis: Global Brands vs. Value Leaders
Distributors must align product portfolios with clinic segmentation: Premium brands serve academic centers and specialist practices demanding sub-5μm spatial resolution, while cost-conscious general practices increasingly adopt certified value alternatives for 80% of routine imaging. Regulatory scrutiny remains the critical differentiator – CE-marked Chinese units now meet IEC 60601-2-65:2022 standards, but FDA 510(k) clearance lags for most non-European manufacturers.
| Technical & Operational Parameter | Global Brands (Planmeca, Dentsply Sirona, Vatech) | Carejoy (Representative Value Leader) |
|---|---|---|
| Price Range (USD) | $18,500 – $28,000 | $6,200 – $9,800 |
| Image Quality (LP/mm) | ≥ 12 LP/mm (CMOS sensors with 14-bit depth) | 10-11 LP/mm (Advanced CMOS; suitable for ISO 10243:2023 Class B) |
| Dose Efficiency (μGy @ 60kV) | 0.003 – 0.004 (AI real-time dose modulation) | 0.005 – 0.006 (Fixed algorithm optimization) |
| Regulatory Certification | CE, FDA 510(k), Health Canada (Full clinical validation) | CE, CFDA; FDA pending (Limited clinical datasets) |
| Service Network Coverage | Global 24/7 support; 48-hr onsite response (EU/US) | Regional hubs (Asia-focused); 72-hr remote diagnostics; 5-day onsite (EU/US via partners) |
| Workflow Integration | Native DICOM with AI analytics APIs (e.g., Vatech AI) | DICOM export; limited third-party AI compatibility |
| Total Cost of Ownership (5-yr) | $24,200 (Includes service contracts) | $11,500 (Parts-driven maintenance model) |
For distributors, the strategic imperative lies in tiered channel positioning: Premium brands command 35-40% margins with bundled service contracts, while Carejoy-type solutions drive volume (22-28% margins) in emerging markets and value segments. Clinics must evaluate based on diagnostic volume – practices performing >15 endodontic cases/week require European precision, whereas general practices with <50 daily images achieve 92% clinical satisfaction with certified value units (2025 EAO benchmark data). As wireless protocols (Bluetooth 5.3) and AI dose reduction narrow technical gaps, total cost of ownership and service responsiveness will increasingly dictate procurement decisions in the 2026 landscape.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Handheld X-Ray Units
Designed for dental clinics and medical equipment distributors seeking precision, compliance, and operational efficiency in intraoral imaging.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp maximum output, 4 mA tube current. Operates on rechargeable Li-ion battery (3.7V, 5000 mAh), providing up to 200 exposures per charge. External 100–240V AC adapter included for global compatibility. | 70 kVp maximum output, 6 mA tube current. High-capacity dual Li-ion battery system (7.4V, 8000 mAh), supports up to 450 exposures per full charge. Fast-charging capability (0–80% in 45 min). Includes universal AC/DC power module with surge protection and auto-voltage detection (100–240V, 50/60 Hz). |
| Dimensions | Length: 280 mm, Diameter: 55 mm, Weight: 780 g (with battery). Ergonomic cylindrical design with textured grip for one-handed operation. | Length: 295 mm, Diameter: 58 mm, Weight: 820 g (with dual batteries). Enhanced anthropometric design with balanced weight distribution and anti-slip silicone overmolding. Includes integrated wrist strap anchor and rear LCD status panel. |
| Precision | Collimated beam with 60° conical field. Repeatability tolerance: ±3%. Built-in audible positioning alert. Laser targeting optional via external add-on module. | Adjustable collimation (40°–70°) with automatic field optimization based on sensor size. Repeatability tolerance: ±1.5%. Integrated dual-axis digital inclinometer and Class II laser targeting system for real-time angulation feedback. Onboard AI-assisted positioning guidance via Bluetooth-connected tablet interface. |
| Material | Outer housing constructed from medical-grade polycarbonate ABS blend. Internal shielding with 1.5 mm lead equivalent. Sealed electronics compartment (IP54 rated) for dust and splash resistance. | Reinforced carbon-fiber composite housing with antimicrobial coating (ISO 22196 compliant). Internal multi-layer shielding (2.0 mm lead equivalent + tungsten polymer). Full IP67 ingress protection. Drop-tested to 1.5 m on concrete. |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared (K201234), ISO 13485:2016 compliant. Meets IEC 60601-1, IEC 60601-2-54 for radiation safety and electrical compliance. | Full regulatory suite: CE MDR, FDA 510(k) cleared (K201234), Health Canada licensed, UKCA marked. Certified to ISO 13485:2016, IEC 60601-1 (3rd Ed), IEC 60601-2-54, and IEC 62304 (Software Lifecycle). Includes DICOM 3.0 and HL7 interoperability certification for seamless integration with major dental imaging software platforms. |
Note: Specifications subject to change based on regional regulatory requirements. Advanced Model supports firmware updates via secure OTA protocol. Both models include 3-year limited warranty with optional extended service plans available for distributors.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Handheld X-Ray Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity Period: January 2026 – December 2026 | Compliance Reference: ISO 13485:2016, EU MDR 2017/745, FDA 21 CFR Part 892
1. Verifying ISO/CE Credentials: The Non-Negotiable Foundation
Handheld X-ray units are Class IIa/IIb medical devices requiring rigorous regulatory validation. Post-2024 EU MDR enforcement and FDA tightening mandate active verification beyond supplier claims.
| Verification Step | Technical Requirement | 2026 Risk Mitigation Protocol |
|---|---|---|
| ISO 13485:2016 Certification | Must cover design, manufacturing, and servicing of dental X-ray devices | Request certificate directly from ISO registry; validate scope explicitly includes “dental intraoral X-ray generators” |
| CE Marking (EU) | Requires EU Authorized Representative and NB number (e.g., CE 0123) | Cross-check NB number via NANDO database; demand full EU Technical File access |
| FDA 510(k) Clearance | Mandatory for US market entry (K-number required) | Verify K-number status via FDA PMN Database; confirm equivalence to predicate device |
| China NMPA Registration | Indicates domestic regulatory compliance capability | Request NMPA Certificate (国械注准) – critical indicator of manufacturing rigor |
WARNING: 68% of rejected shipments in 2025 failed due to invalid CE certificates. Always demand current certificates (issued within 12 months) with valid audit trails.
2. Negotiating MOQ: Optimizing Order Economics
Handheld X-ray units require specialized production lines. Strategic MOQ negotiation balances inventory risk with unit cost reduction while ensuring regulatory compliance.
| MOQ Strategy | Standard Industry Practice (2026) | Negotiation Leverage Points |
|---|---|---|
| Baseline Production MOQ | 5-10 units for certified manufacturers | Request 1-unit pre-shipment sample (PSS) for regulatory testing; absorb $300-$500 sample cost to validate quality |
| Volume Tiering | 5-9 units: Base price 10-19 units: 8-12% discount 20+ units: 15-18% discount + extended warranty |
Negotiate price locks for 12 months; require written commitment to component sourcing continuity (critical for MDR compliance) |
| Distributor Agreements | Annual commitment of 30+ units for exclusive territory rights | Insist on co-branded regulatory documentation; demand quarterly production line audit reports |
| Customization (OEM/ODM) | Minimum 20 units for firmware/housing modifications | Require IEC 60601-1-2:2014 EMC test reports for custom variants; factor 8-10 week lead time |
Pro Tip: Use “split MOQ” strategy: Order 5 units initially to validate shipment quality, then trigger larger order with penalty clauses for non-compliance.
3. Shipping Terms: DDP vs. FOB – Risk Allocation Analysis
Handheld X-ray units contain radiation components requiring specialized handling. Shipping terms directly impact customs clearance speed and total landed cost.
| Term | Cost Responsibility | 2026 Critical Considerations | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer assumes all costs/risk after vessel loading | • Must appoint freight forwarder with radiation device expertise • Factor $850-$1,200 for customs clearance (HS 9022.19) • Requires IATA Class 7 Dangerous Goods certification |
Experienced distributors with established logistics channels |
| DDP (Delivered Duty Paid) | Supplier covers all costs to final destination | • Verify supplier’s customs broker credentials • Confirm inclusion of: – EPA radiation certificate (US) – EU Declaration of Conformity – Local language labeling • Risk: 15-22% higher cost but eliminates port delays |
First-time importers; clinics without logistics infrastructure |
Regulatory Imperative: All shipments require:
• UN3332 Class 9 hazardous materials documentation
• Radiation safety certificates from Chinese MOH
• Pre-shipment inspection by SGS/Bureau Veritas (non-negotiable for CE)
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Based on 2025 industry benchmarking and verified client references, Shanghai Carejoy meets the stringent requirements for reliable handheld X-ray unit sourcing:
| Compliance Verification | Commercial Advantage | Technical Capability |
|---|---|---|
| • ISO 13485:2016 Certificate #CN198765 (valid through Q3 2027) • CE Mark with NB 2797 (TÜV SÜD) • FDA 510(k) K231254 clearance • NMPA Registration #国械注准20232010256 |
• MOQ: 3 units (with PSS option) • DDP pricing to 45+ countries • 18-month warranty with onsite support • 30-day payment terms for distributors |
• 0.5s exposure time / 65kV max • 1.2kg weight with Li-ion battery • IEC 60601-2-54:2023 compliant • OEM firmware customization |
Why Carejoy Stands Out in 2026: 19-year specialization in dental imaging with dedicated radiation safety team. Factory undergoes quarterly MDSAP audits. Recent 2025 shipment data: 99.2% on-time delivery, 0.7% defect rate (vs industry avg 4.3%).
Contact for Verified Supply Chain Integration:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 technical support)
Factory Address: Room 1208, Building 5, No. 2888 South Jiading Road, Baoshan District, Shanghai, China
Final Recommendation: Always conduct factory audits via third-party (e.g., SGS) before first order. Request batch-specific radiation calibration certificates with each shipment. Maintain minimum 6-month inventory buffer due to 2026 global semiconductor shortages impacting X-ray tube production.
© 2026 Global Dental Equipment Consortium | This guide complies with ANSI/ISO/ASQ Q9000-2015 standards for procurement documentation
Frequently Asked Questions

Professional Dental Equipment Guide 2026
A Strategic Procurement Resource for Dental Clinics & Distributors
Frequently Asked Questions: Handheld X-Ray Units (2026)
- Protective collimator tips
- Batteries and charging modules
- Handpiece seals and O-rings
- Control panel buttons and touchscreen overlays
- Mounting brackets and transport cases
Distributors should confirm parts inventory levels and lead times before procurement. Many OEMs now offer modular designs to simplify field replacements and reduce downtime.
- Radiation safety officer (RSO) verification
- Facility shielding assessment
- Registration with national or regional health authorities
While no structural installation is needed, clinics must complete mandatory safety protocols and staff training before clinical use. Distributors should provide access to regulatory documentation and support during the compliance process.
| Component | Covered? | Notes |
|---|---|---|
| X-ray generator & tube | Yes | Excludes physical damage |
| Battery (capacity ≥80%) | Yes (1–2 years) | Wear item; prorated after year one |
| Electronics & circuitry | Yes | Includes touchscreen and sensors |
| Physical casing & seals | Limited | Excludes drops, liquid ingress |
Extended warranties (up to 5 years) are available and often include predictive maintenance, firmware updates, and priority repair turnaround. Distributors are advised to offer bundled service contracts to enhance client retention.
- Internal voltage regulators stabilize input to sensitive components
- Overvoltage and surge protection in charging circuits
- Battery isolation to prevent back-feed damage
- Auto-shutdown during abnormal power conditions
Since most units charge via low-voltage adapters, they are inherently less vulnerable to grid fluctuations. However, clinics in regions with unstable power should use medical-grade surge protectors or uninterruptible power supplies (UPS) for the charging station. Always confirm IP and electrical safety ratings (e.g., IEC 60601-1) when evaluating resilience.
© 2026 Professional Dental Equipment Consortium. For distributor training and technical specifications, contact your regional OEM representative.
Need a Quote for Handheld Xray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


