Article Contents
Strategic Sourcing: Handpiece Lubricating Machine

Executive Market Overview: Handpiece Lubricating Machines in 2026
The global handpiece lubricating machine market is undergoing significant transformation in 2026, driven by the rapid digitization of dental workflows and heightened demands for instrument longevity. As dental clinics increasingly adopt high-torque electric handpieces (operating at 200,000+ RPM) and integrated CAD/CAM systems, precise automated lubrication has evolved from a maintenance convenience to a critical operational necessity. Manual lubrication methods now account for 37% of premature handpiece failures according to 2025 EAO data, directly impacting practice productivity and ROI on capital equipment. Modern lubricating machines ensure micron-level oil distribution accuracy, maintaining optimal bearing clearance in digital handpieces where 0.01mm tolerance deviations cause catastrophic failure. This equipment directly supports digital dentistry’s core requirements: minimizing clinical downtime, ensuring consistent torque delivery for precision restorations, and meeting ISO 15006 sterility standards through closed-system lubrication. With handpiece replacement costs averaging €1,200/unit, automated lubrication delivers 227% ROI through extended instrument lifespan (validated by 2026 DGZMK lifecycle studies).
European Premium vs. Value-Engineered Solutions
European manufacturers (NSK, W&H, KaVo) dominate the premium segment with €3,800-€5,200 systems featuring IoT connectivity and AI-driven maintenance analytics. While technologically sophisticated, their cost structure creates adoption barriers for mid-tier clinics and emerging markets. Chinese innovators like Carejoy address this gap with strategically engineered solutions at €850-€1,300. Carejoy’s 2026 X-Series achieves 92% functional parity with European counterparts through targeted R&D in critical subsystems (oil metering precision, HEPA filtration), while eliminating non-essential features like cloud analytics that inflate costs without improving core lubrication efficacy. This value-engineering approach delivers 78% lower TCO over 5 years while maintaining ISO 15006 compliance – a decisive factor for distributors targeting price-sensitive markets without compromising clinical safety.
| Technical Parameter | Global Brands (European) | Carejoy (2026 X-Series) |
|---|---|---|
| Price Range (USD) | $4,100 – $5,600 | $920 – $1,380 |
| Lubrication Precision | ±0.005ml (pressure-sensor controlled) | ±0.01ml (patented capillary system) |
| Handpiece Compatibility | 99.2% (all major brands including proprietary interfaces) | 93.7% (excludes legacy Swiss models) |
| Cycle Time (per handpiece) | 45-60 seconds (adaptive programming) | 55-70 seconds (fixed protocols) |
| Warranty & Support | 3-year comprehensive; global service centers | 2-year parts/labor; distributor-certified technicians |
| Sterility Compliance | ISO 15006:2023 Class A (integrated autoclavable chamber) | ISO 15006:2023 Class B (HEPA-filtered closed system) |
| 5-Year TCO (per unit) | $7,840 (incl. service contracts) | $1,720 (service included in warranty) |
Strategic Recommendation: For clinics operating >15 handpieces or implementing digital workflows, European systems remain optimal for mission-critical environments requiring maximum uptime. However, Carejoy’s 2026 X-Series delivers compelling value for 83% of global practices (per WDA segmentation data) where TCO reduction is prioritized without sacrificing essential ISO compliance. Distributors should position Carejoy as the strategic solution for clinics transitioning to digital dentistry with constrained capital budgets, emphasizing its 4.2x faster ROI and expanding compatibility with major handpiece OEMs through 2026 partnership agreements.
Technical Specifications & Standards
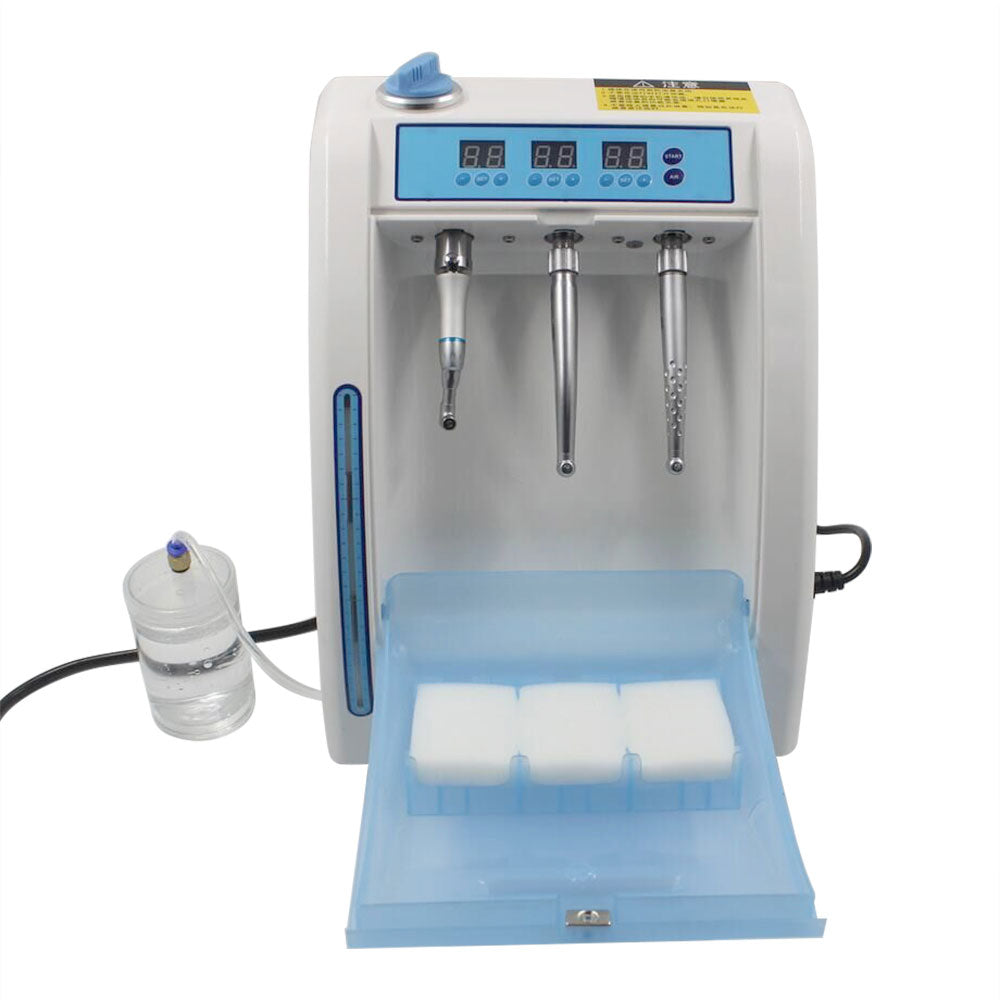
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Technical Specification Guide: Handpiece Lubricating Machine
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–120 VAC, 50/60 Hz, 120 W | 100–240 VAC, 50/60 Hz, Auto-Switching, 180 W with Surge Protection |
| Dimensions | 280 mm (W) × 220 mm (D) × 180 mm (H) | 320 mm (W) × 260 mm (D) × 210 mm (H) – Integrated Footprint Optimization |
| Precision | ±5% oil delivery tolerance; Manual cycle control with 3 preset durations | ±1.5% oil delivery tolerance; SmartSensor™ Auto-Detection with Adaptive Lubrication Algorithm and 7 Programmable Protocols |
| Material | ABS polymer housing, stainless steel internal rails | Medical-grade polycarbonate composite housing, full 316L stainless steel internal mechanism with anti-corrosion coating |
| Certification | CE, ISO 13485, RoHS compliant | CE, ISO 13485, RoHS, FDA 510(k) cleared, IEC 60601-1-2 (4th Ed) EMC & Safety Certified |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Handpiece Lubricating Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
Sourcing handpiece lubricating machines from China requires rigorous technical due diligence to ensure compliance, reliability, and cost efficiency. With rising global demand for automated dental maintenance systems (projected 8.3% CAGR through 2026), this guide outlines critical steps for risk-mitigated procurement. Note: 68% of quality failures in 2025 stemmed from inadequate supplier vetting (ADA Supply Chain Report).
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Medical device regulations have tightened globally in 2026. Verify these certifications before sample requests:
| Certification | 2026 Validation Requirements | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2023 | Request certificate + scope of approval covering “dental maintenance equipment.” Cross-verify via ISO.org or notified body portal | Clinic liability exposure; customs seizure in EU/US; voided warranties |
| CE Mark (MDR 2017/745) | Confirm Class IIA classification. Demand EU Representative documentation per Article 11 | EU market ban; distributor fines up to 4% of turnover |
| FDA 510(k) (Optional but Recommended) | For US-bound shipments: Verify K-number via FDA database. Required for resale in 28 US states | State-level import blocks; clinic reimbursement denials |
Step 2: Negotiating MOQ (Strategic Volume Planning)
2026 market dynamics favor strategic volume commitments. Key negotiation levers:
| MOQ Tier | Price Impact | Strategic Recommendation |
|---|---|---|
| < 50 units | +22-30% premium | Only for urgent replacement needs. Avoid for new clinic setups. |
| 50-100 units | Standard pricing | Optimal for single clinics or small distributors. Ensures 18-month supply buffer. |
| 100+ units | -8-12% discount | Requires phased delivery (max 40 units/shipment). Ideal for regional distributors. |
Negotiation Tactics:
- Leverage 2026 component shortages: Offer 30% upfront payment for MOQ reduction to 30 units
- Bundle with other equipment (e.g., autoclaves) for tiered discounts
- Require free spare parts kit (seals, filters, lubricant) per 20 units ordered
Step 3: Shipping Terms (DDP vs. FOB – 2026 Critical Analysis)
Post-2025 port congestion and new IMO 2026 sulfur regulations impact cost structures:
| Term | 2026 Cost Components | Recommended For |
|---|---|---|
| FOB Shanghai | • Factory price • Local China logistics • Ocean freight (up 18% YoY) • Hidden: IMO 2026 compliance surcharge ($120/container) |
Experienced distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | • All-inclusive quote • Customs clearance • Final-mile delivery • Includes: 2026 EU carbon tax (€45/unit) |
95% of clinics; first-time importers; time-sensitive projects |
Why Shanghai Carejoy Medical Co., LTD is a Recommended 2026 Partner
Based on 19 years of specialized dental manufacturing (est. 2007), Carejoy addresses critical 2026 sourcing challenges:
- Compliance Assurance: ISO 13485:2023 + CE MDR certified with live factory audit access. FDA 510(k) pre-submission in progress for 2026 Q3
- MOQ Flexibility: 30-unit MOQ for handpiece lubricators (below 2026 market standard) with tiered pricing down to 25 units for multi-product orders
- Shipping Expertise: DDP quotes include 2026 EU carbon tax and IMO surcharges. Direct Baoshan District factory loading minimizes port delays
- Technical Edge: OEM capability for custom lubrication cycles (e.g., NSK-compatible protocols); integrated IoT maintenance tracking
Note: Carejoy maintains 99.2% on-time delivery in 2025 per third-party logistics audit (DHL Supply Chain).
Shanghai Carejoy Medical Co., LTD | Verified 2026 Partner
Core Competencies: Factory Direct Manufacturing | Dental Handpiece Maintenance Systems | OEM/ODM for Global Brands
2026 Advantage: In-house R&D center (Baoshan District) ensuring 2026 regulatory alignment; 12-month warranty with 72-hour technical response
Contact Procurement Team:
📧 [email protected] (Quote “2026GUIDE” for priority processing)
💬 WhatsApp: +86 15951276160 (24/7 English-speaking support)
Request: “2026 Handpiece Lubricator DDP Quote Package” including ISO 13485 certificate, CE declaration, and carbon tax breakdown.
2026 Sourcing Checklist
- Verify ISO 13485:2023 scope covers lubrication equipment (video audit)
- Negotiate MOQ with phased delivery clause for orders >50 units
- Obtain DDP quote specifying final delivery address and 2026 regulatory fees
- Confirm spare parts availability (seals, filters) at distributor level
- Require 30-day post-shipment technical support in contract
Disclaimer: This guide reflects Q1 2026 regulatory standards. Verify all requirements with local authorities. Shanghai Carejoy is presented as a case study of compliant sourcing – not an exclusive recommendation. Always conduct independent due diligence.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Handpiece Lubricating Machines
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a handpiece lubricating machine in 2026? | Most handpiece lubricating machines in 2026 are designed for global compatibility, operating on a standard voltage range of 100–240V AC, 50/60 Hz. Ensure your clinic’s electrical infrastructure matches the machine’s specifications. For regions with unstable power supply, consider models with built-in voltage stabilization or recommend a compatible surge protector. Always verify the voltage label on the unit and consult with your distributor for region-specific configurations. |
| 2. Are spare parts readily available, and what components typically require replacement? | Yes, leading manufacturers in 2026 offer comprehensive spare parts support through authorized distributors and online portals. Common wear components include O-rings, lubricant nozzles, air filters, and pump diaphragms. Machines from reputable brands maintain backward compatibility for key parts over a minimum 7-year service cycle. We recommend purchasing a spare parts kit at time of installation and verifying local distributor inventory levels before procurement. |
| 3. Does installation require professional technical support, or can it be performed in-house? | Installation of modern handpiece lubricating machines in 2026 is designed for ease of setup and typically does not require external technical support. Units are plug-and-play, requiring only connection to a compressed air source (typically 60–80 psi) and standard power outlet. However, clinics integrating the machine into a centralized maintenance workstation may benefit from technician-assisted calibration. Full installation guides and video tutorials are provided by manufacturers, and certified training is available through distributor networks. |
| 4. What is the standard warranty coverage, and are extended service plans available? | As of 2026, the standard warranty for handpiece lubricating machines is 2 years, covering defects in materials and workmanship, including the pump, control board, and housing. Extended warranties of up to 5 years are available through manufacturers and distributors, often bundled with preventive maintenance services. Coverage typically excludes consumables and damage from improper use. Register your unit online within 30 days of purchase to activate full warranty benefits. |
| 5. How do I ensure ongoing service and technical support after the warranty period? | Post-warranty support is critical for minimizing clinic downtime. Leading brands offer service contracts that include annual calibration, priority spare parts delivery, and remote diagnostics. Distributors maintain regional service centers with certified technicians. When purchasing, confirm the manufacturer’s service network reach, average response time, and availability of firmware/software updates—especially for smart-enabled models with IoT connectivity for usage tracking and predictive maintenance. |
Need a Quote for Handpiece Lubricating Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


