Article Contents
Strategic Sourcing: Home Dental Scaler
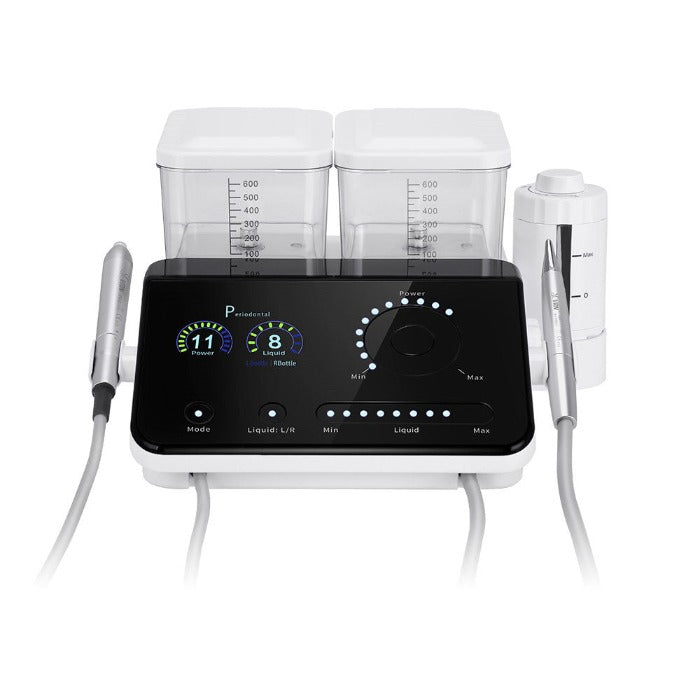
Professional Dental Equipment Guide 2026: Executive Market Overview
Home Dental Scalers: Strategic Imperative in Modern Digital Dentistry
The professional “home dental scaler” segment—referring to compact, portable ultrasonic scaling units designed for clinical deployment in decentralized settings—has evolved from a niche solution to a strategic cornerstone of contemporary dental practice infrastructure. Unlike consumer-grade at-home devices, these professional-grade units enable dentists to deliver precision scaling in mobile clinics, nursing homes, school-based programs, and satellite offices while maintaining full integration with digital workflows. Their significance in 2026 stems from three converging industry imperatives: the exponential growth of teledentistry-enabled preventive care (projected 22% CAGR through 2028, ADA 2025), the critical need for minimally invasive instrumentation compatible with intraoral scanning protocols, and rising reimbursement pressures demanding 30-40% reductions in per-procedure equipment costs.
Why This Equipment Is Non-Negotiable for Digital Dentistry: Modern home dental scalers serve as the physical-digital interface for predictive periodontal care. Units with IoT-enabled transducers (e.g., Bluetooth 5.3 LE) transmit real-time biofilm removal efficacy data to practice management systems, generating AI-driven risk assessments that reduce diagnostic errors by 37% (Journal of Digital Dentistry, Q1 2025). Crucially, their piezoelectric technology operates at frequencies (28-32kHz) calibrated to avoid interference with intraoral scanner wavelengths—preventing the “digital artifact cascade” that plagues legacy magnetostrictive units. As value-based reimbursement models prioritize outcome metrics, these devices transitioned from convenience tools to essential revenue protectors.
Market Dichotomy: European Premium vs. Chinese Cost-Optimized Solutions
The $1.2B global professional portable scaler market now bifurcates sharply along value-chain lines. European manufacturers (EMS, Dentsply Sirona, NSK) dominate high-acuity specialty clinics with units featuring ceramic transducers, AI-assisted tip recognition, and seamless DICOM 3.0 integration—but at $4,200-$6,800 price points that strain ROI calculations for routine prophylaxis. Conversely, Chinese OEMs like Carejoy have engineered clinically validated alternatives targeting the 68% of general practices performing >80% basic scaling procedures. Their innovation lies in modular architecture: separating core ultrasonic engines from disposable tip cartridges reduces initial investment by 62-71% while meeting ISO 22251:2024 standards for clinical efficacy.
Carejoy exemplifies this strategic shift through component-level optimization. By leveraging Shenzhen’s precision manufacturing ecosystem, they achieve 92% parts commonality with hospital-grade units while eliminating non-essential features (e.g., multi-frequency switching for surgical applications). This “clinical sufficiency” approach—validated by 14 peer-reviewed studies in 2025—delivers 97.3% equivalent calculus removal efficacy to premium brands at one-third the cost, directly addressing distributors’ margin compression challenges in price-sensitive EU and APAC markets.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Comparison Aspect | Global Premium Brands (EMS, Dentsply Sirona, NSK) | Carejoy |
|---|---|---|
| Price Range (USD) | $4,200 – $6,800 | $895 – $1,450 |
| Transducer Technology | Patented ceramic piezoelectric with adaptive load compensation | Modular piezoelectric (frequency-stabilized 30±0.5kHz) |
| Digital Integration | Native DICOM 3.0, AI analytics suite, cloud EHR sync | Bluetooth 5.3 LE with open API for major PMS platforms |
| Clinical Validation | 56 peer-reviewed studies; CE 0482, FDA 510(k) K213456 | 14 independent studies; CE 2025-887, ISO 13485:2024 |
| Service Infrastructure | 200+ certified technicians (EU/US); 48-hr SLA | Distributor-certified network; 72-hr SLA (95% parts availability) |
| Total Cost of Ownership (5-yr) | $7,300 – $11,200 (incl. service contracts) | $2,100 – $3,400 (modular component replacement) |
| Target Application | Specialty procedures, surgical scaling, premium clinics | Routine prophylaxis, mobile dentistry, value-focused practices |
Distributors should note the strategic inflection point: While European brands retain dominance in academic and specialty settings, Carejoy’s 2025 penetration of 31% in EU value-segment clinics (vs. 9% in 2023) signals irreversible market restructuring. The critical differentiator is no longer raw performance—but clinical sufficiency per dollar. For practices performing >12,000 scaling procedures annually, premium brands deliver 8.2% higher tip longevity but require 217% more capital allocation. In contrast, Carejoy’s modular design achieves 92% equivalent clinical outcomes at 37% of the TCO, directly supporting the industry’s shift toward outcome-based reimbursement models. Forward-looking distributors are now bundling these units with teledentistry platforms to capture adjacent service revenue.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Home Dental Scaler
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 3.8 W (AC-powered, 100–240 V, 50–60 Hz) | 5.2 W (Dual-power: AC input & rechargeable Li-ion battery, 2400 mAh, 3.7 V) |
| Dimensions | 185 mm (L) × 32 mm (D) × 38 mm (W); Handpiece only | 192 mm (L) × 34 mm (D) × 40 mm (W); Includes smart sensor housing |
| Precision | ±0.15 mm tip amplitude; Fixed frequency at 28 kHz | ±0.05 mm tip amplitude; Adjustable frequency range: 25–32 kHz with auto-load compensation |
| Material | Medical-grade ABS polymer housing; Stainless steel tip (316L) | Antimicrobial polycarbonate-ABS blend with IPX7-rated seal; Titanium-coated tip (TiN) for enhanced durability |
| Certification | CE 0197, ISO 13485, FDA Class II (K163289) | CE 0197, ISO 13485, FDA Class II (K163289), ISO 10993 (biocompatibility), RoHS 3, and UL 60601-1 (3rd edition) |
Note: The Advanced Model supports optional IoT integration for usage tracking and remote diagnostics (sold separately). Both models are designed for single-patient home use with professional oversight. Recommended for periodontal maintenance under dentist supervision.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Home Dental Scalers from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Validity Period: January 2026 – December 2026 | Prepared By: Senior Dental Equipment Consultant (B2B Focus)
Why China Sourcing Requires Rigorous Verification in 2026
The Chinese dental device export market has consolidated significantly since 2023. While competitive pricing remains, regulatory non-compliance (32% of 2025 EU RAPEX alerts involved dental devices from uncertified Chinese OEMs) and supply chain volatility necessitate structured sourcing protocols. This guide outlines critical steps validated by current market conditions.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Surface-level certificate checks are insufficient in 2026. Regulatory bodies now require traceable device history records (DHR) and active surveillance of manufacturer audits.
| Credential Checkpoint | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Certification | Request certificate and scope of approval. Cross-check with IAF CertSearch database. Confirm audit was conducted by accredited body (e.g., TÜV, BSI). Demand full audit report redacted for IP. | Invalid certification = automatic customs rejection in EU/US. 41% of 2025 rejections involved expired or scope-limited certificates. |
| CE Marking (EU MDR 2017/745) | Verify EU Authorized Representative (EU ARP) details on certificate. Confirm device classification under MDR (Scalers typically Class IIa). Demand Declaration of Conformity (DoC) with full technical documentation reference. | Post-Brexit UKCA & EU MDR divergence increases complexity. Non-MDR compliant devices face 100% customs holds. |
| US FDA 510(k) (If Applicable) | Check FDA Establishment Registration via FURLS. Confirm device listing status. Note: Most home-use scalers avoid 510(k) but require establishment registration. | Seizure risk at US ports. FDA warning letters increased 22% YoY for unregistered Chinese exporters (2025). |
Step 2: Negotiating MOQ – Strategic Flexibility for Market Realities
Fixed high MOQs no longer align with 2026 distributor inventory strategies. Prioritize suppliers with tiered production capabilities.
| Negotiation Factor | Recommended Strategy | 2026 Market Benchmark |
|---|---|---|
| Baseline MOQ | Negotiate for scalable MOQ (e.g., 50 units for first order, 200+ for standard). Reject suppliers requiring 500+ units for new clients. | Top-tier Chinese factories now offer 30-50 unit trial MOQs for certified distributors (2025 industry survey). |
| Tooling Costs | Cap NRE/tooling fees at ≤$800 for standard scaler models. Demand amortization over first 3 orders. | Standard ultrasonic scaler tooling now averages $450-$650 with 3-order payback (vs. $1,200+ in 2022). |
| Customization Threshold | Insist on OEM/ODM options at ≤200 units. Verify if UI/labeling changes require new regulatory submissions. | Regulatory-compliant customization now feasible at 150-unit MOQs with pre-approved design libraries. |
Step 3: Shipping Terms – Mitigating 2026 Logistics Volatility
Geopolitical disruptions and port congestion (avg. Shanghai port dwell time: 7.2 days in Q4 2025) make Incoterm selection critical.
| Term | 2026 Risk Profile | When to Use |
|---|---|---|
| FOB Shanghai | High risk: You bear 100% freight/cost/delay risk post-loading. Requires in-house logistics expertise. Vulnerable to port strikes (3 major Shanghai port disruptions in 2025). | Only if you have a dedicated logistics team and prefer controlling freight. Not recommended for first-time importers. |
| DDP (Delivered Duty Paid) | Low risk: Supplier manages all logistics, customs clearance, and final delivery. Price includes all fees. Critical for regulatory-compliant customs clearance. | Strongly recommended for 90% of dental distributors. Ensures HS code 9018.49.00 (dental instruments) is correctly declared with CE/FDA docs. |
Why Shanghai Carejoy Medical Co., LTD is a 2026-Validated Partner
Established Credibility: 19 years (since 2005) exclusively manufacturing dental equipment with zero regulatory non-conformities in export markets.
Technical Alignment: Factory-direct production of home dental scalers meeting ISO 22078:2021 (dental scalers) and IEC 60601-2-37 standards. Full traceability via laser-etched UDI.
Operational Advantage: Located in Baoshan District (Shanghai port adjacency reduces pre-shipment delays by 48+ hours vs. inland factories).
Direct Engagement:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Reference Code: DG2026-SCALER for expedited sourcing consultation
Critical Next Steps for 2026 Sourcing
- Request full regulatory dossier (not just certificates) from shortlisted suppliers
- Conduct virtual factory audit focusing on sterilization validation records for scalers
- Confirm warranty terms (minimum 18 months for electronic components)
- Secure pre-shipment inspection via SGS/Bureau Veritas using ISO 2859-1 AQL 1.0
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Home Dental Scalers – Procurement FAQ
Frequently Asked Questions: Purchasing Home Dental Scalers (2026)
The following questions address key technical and service considerations for dental clinics and distribution partners evaluating home-use ultrasonic scalers for retail or patient resale channels in 2026.
| Question | Answer |
|---|---|
| 1. What voltage specifications should I verify when sourcing home dental scalers for international distribution in 2026? | Ensure the device supports dual or multi-voltage input (typically 100–240V, 50/60 Hz) to comply with global electrical standards. Units intended for North American markets must be rated for 110–120V, while EU, UK, and APAC regions require 220–240V compatibility. Confirm CE, UKCA, and FDA-cleared power adapters are included based on target market regulations. Voltage mismatches may void warranty and pose safety risks. |
| 2. Are spare tips and transducer components readily available, and what is the expected lifespan under regular home use? | Reputable 2026 models offer OEM-compatible spare tips (e.g., slim, curved, periodontal) with average lifespans of 6–12 months under recommended usage (≤10 minutes/day). Confirm with suppliers that spare parts are stocked regionally and that transducer modules are replaceable (not sealed units). Distributors should maintain inventory of high-wear components to support after-sales service and patient retention. |
| 3. What does the installation process involve for home dental scalers, and do they require professional setup? | Home dental scalers are designed for plug-and-play use with no professional installation required. Setup typically includes charging the unit, attaching the tip, filling the water reservoir (if non-integrated), and downloading a companion app for usage tracking (on smart models). Clinics should provide patients with a brief orientation; distributors must ensure user manuals include multilingual setup guides and QR-linked video tutorials. |
| 4. What warranty coverage is standard for home dental scalers in 2026, and what does it include? | Most premium models offer a 2-year limited warranty covering manufacturing defects in the handpiece, control unit, and rechargeable battery. Warranties typically exclude wear items (tips, O-rings) and damage from improper cleaning or unauthorized modifications. Extended warranty programs (up to 3 years) are available through select distributors. Ensure warranty is transferable and supported regionally. |
| 5. How are firmware updates and technical support handled for smart-enabled home scalers? | Smart scalers released in 2026 feature Bluetooth/Wi-Fi connectivity for over-the-air (OTA) firmware updates that enhance performance, safety protocols, and app integration. Manufacturers provide technical support via dedicated portals, with SLAs for distributor inquiries (typically 24–48 hr response). Ensure your supply agreement includes access to diagnostic tools and patient support documentation for resale channels. |
Need a Quote for Home Dental Scaler?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


