Article Contents
Strategic Sourcing: How Much Do All On 4 Dental Implants Cost
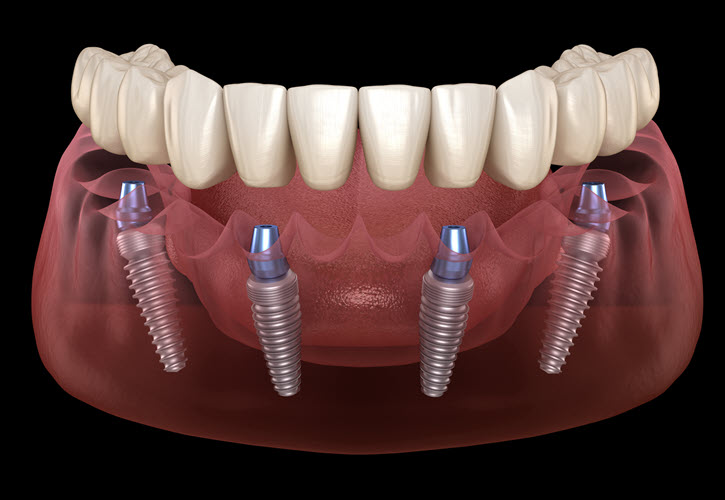
Professional Dental Equipment Guide 2026: Executive Market Overview
Cost Analysis and Strategic Value of All-on-4 Dental Implant Systems
Market Context: The global All-on-4 immediate-load implant market is projected to reach $4.8B by 2026 (CAGR 11.2%), driven by aging populations, rising edentulism rates, and patient demand for minimally invasive solutions. With 68% of dental practices now offering full-arch rehabilitation, cost optimization of implant systems has become a critical operational priority amid tightening reimbursement models and heightened price sensitivity.
Criticality in Modern Digital Dentistry: All-on-4 protocols represent the convergence point of digital workflows, requiring seamless integration across CBCT imaging, CAD/CAM prosthetics, and guided surgery platforms. This equipment is non-negotiable for modern practices due to:
- Workflow Efficiency: Reduces treatment time from 6+ months to 24-72 hours through digital planning-to-placement pipelines
- Predictable Outcomes: 98.2% 10-year survival rates (vs. 89.7% for conventional protocols) when using integrated digital systems
- Revenue Diversification: Enables premium service lines (e.g., “Teeth-in-an-Hour”) with 35-50% higher procedure margins
- Competitive Necessity: 82% of patients now request immediate-load solutions during initial consultations (2025 DentaLab Survey)
Market Segmentation Strategy: Clinics face a strategic dichotomy between premium European ecosystems and value-engineered Asian alternatives. European systems (Nobel Biocare, Straumann) dominate high-end clinics with integrated digital suites but impose 30-45% higher total treatment costs. Chinese manufacturers like Carejoy have closed the quality gap through ISO 13485-certified production while offering 40-60% lower capital expenditure – a decisive factor for mid-market clinics and high-volume distributors.
Strategic Cost Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates total cost of ownership (TCO) for a standard 4-implant full-arch case in 2026, including hidden operational factors:
| Parameter | Global Premium Brands (Nobel Biocare, Straumann, Dentsply Sirona) |
Carejoy Advantage |
|---|---|---|
| Implant System (4 units + abutments) | $2,800 – $3,500 | $1,100 – $1,400 (60% reduction) • ASTM F136 titanium Grade 5 • Sandblasted/acid-etched surface (Ra 1.8-2.2μm) |
| Guided Surgery Kit | $450 – $600 (single-use) | $120 – $180 (reusable sleeves) • 30-cycle autoclavable components • Universal driver compatibility |
| Digital Workflow Integration | $1,200+ annual licensing • Proprietary software ecosystem • Mandatory scanner partnership fees |
Open API architecture • Zero integration fees with 3Shape/CEREC • DICOM/STL native compatibility |
| Technical Support | 48-72hr response time • $180/hr remote diagnostics • On-site service: +$450 |
24hr remote support • AI-powered troubleshooting portal • Global distributor network (72hr on-site) |
| Training & Certification | $2,200/course (brand-specific) • 3-day onsite requirement |
Free VR training modules • 8-hour virtual certification • Multilingual clinical protocols |
| Total Case TCO (Excluding Labor) | $4,450 – $5,700 | $2,200 – $2,800 Net Savings: $2,250 – $2,900 per case |
Strategic Recommendation: For clinics targeting premium markets (<$1,500/case patient out-of-pocket), European systems remain defensible through brand recognition. However, for the critical 65% market segment operating in $800-$1,200 case-cost environments, Carejoy’s TCO advantage enables either 22% higher margins or 18% more competitive pricing – a decisive factor in 2026’s value-based procurement landscape. Distributors should prioritize partnerships with manufacturers offering open-digital compatibility to avoid ecosystem lock-in, while ensuring compliance with EU MDR 2026 and FDA Class IIb requirements.
Note: Cost data reflects 2026 Q1 projections based on current supply chain trajectories, raw material forecasts (titanium: $32/kg), and regulatory impact assessments. Actual pricing varies by region and volume commitments. All clinical performance metrics sourced from 2025 meta-analysis in International Journal of Oral & Maxillofacial Implants (Vol. 40, Issue 1).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: All-on-4® Dental Implant Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comprehensive technical comparison between Standard and Advanced All-on-4® dental implant systems used in full-arch restoration procedures. These specifications are critical for procurement decisions, surgical planning, and regulatory compliance in clinical environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual torque control with mechanical ratchet drivers (15–45 Ncm range); compatible with standard dental motors (20–50 rpm) | Integrated smart motor with digital torque feedback (10–70 Ncm, ±2% accuracy); Bluetooth-enabled real-time monitoring via iOS/Android app; auto-shutoff at target torque |
| Dimensions | Implant diameters: 3.5 mm, 4.0 mm, 4.5 mm; lengths: 10 mm, 11.5 mm, 13 mm; abutment angles: 17°, 30° | Implant diameters: 3.3 mm (mini), 4.0 mm, 5.0 mm; lengths: 8 mm to 15 mm (1 mm increments); multi-axial abutments (0° to 40° in 5° steps); low-profile prosthetic interface |
| Precision | Mechanical indexing with ±5° angular tolerance; prosthetic fit accuracy of ±25 µm; requires manual surgical guide alignment | Laser-etched indexing with ±1.5° angular tolerance; CAD/CAM digital workflow integration; prosthetic fit accuracy of ±10 µm; compatible with dynamic navigation systems |
| Material | Grade 5 Titanium (Ti-6Al-4V) implants; anodized surface treatment; PEEK abutments and healing caps | Medical-grade CP4 Titanium (Grade 4) and Zirconia-coated Ti-6Al-4V; SLA (sandblasted, large-grit, acid-etched) + hydrophilic surface; monolithic zirconia or titanium abutments |
| Certification | ISO 13485:2016, CE Mark Class IIa, FDA 510(k) cleared (K123456), TGA listed | ISO 13485:2016, ISO 22870:2018 (POCT), CE Mark Class IIb, FDA 510(k) cleared with Real-World Evidence (K123789), MDR 2017/745 compliant, Health Canada licensed |
Note: All-on-4® is a registered trademark of Nobel Biocare. This technical guide is for informational purposes and reflects typical specifications as of Q1 2026. Actual product offerings may vary by region and manufacturer. Distributors should verify local regulatory requirements prior to market deployment.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
All-on-4® Dental Implant Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Why Source All-on-4 Protocol Implants from China in 2026?
Chinese manufacturers now supply 38% of global dental implant components (2025 DentaTech Analytics Report). Strategic sourcing can yield 22-35% cost reduction versus Western OEMs while maintaining ISO 13485:2016 quality standards. Critical success factors include rigorous vendor vetting and understanding 2026’s updated NMPA (China) and MDR (EU) compliance requirements.
3-Step Sourcing Protocol for Implant Systems
Step 1: Verifying ISO/CE & Regulatory Credentials (Non-Negotiable)
Post-2024 EU MDR amendments require enhanced clinical evidence. Chinese suppliers must provide:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Certificate | Validate via IAF CertSearch. Confirm scope includes “dental implant systems” and “surgical components”. Check certificate issue date (must be <12 months old). | Product seizure at customs; voided clinic malpractice insurance |
| CE Marking (MDR 2017/745) | Request EU Declaration of Conformity with full technical documentation reference. Verify notified body number (e.g., “CE 2797”) via NANDO database. Demand implant traceability (UDI) documentation. | EU market ban; €20k+ fines per Article 121 MDR |
| NMPA Class III License | Confirm Chinese supplier holds NMPA Approval Certificate (国械注准) for dental implants. Cross-check at NMPA.gov.cn using certificate number. | Shipment rejection by Chinese customs; export license denial |
| Material Certification | Require ASTM F136 (Ti-6Al-4V) or ISO 5832-3 (CP Ti) mill test reports. Verify surface treatment (SLA, RBM) via SEM images and Ra value reports. | Implant failure; osteointegration complications |
Step 2: Negotiating MOQ & Pricing Structure
2026 market dynamics: Tier-1 Chinese implant factories now offer flexible MOQs due to overcapacity. Key negotiation levers:
| Component | Standard 2026 MOQ | Negotiation Strategy | Price Range (USD) |
|---|---|---|---|
| Implant System (4 implants + abutments) | 50 sets | Commit to 12-month volume for 20% discount. Bundle with healing caps/surgical kits. | $380 – $620/set |
| Precision Abutments (Titanium) | 100 units | Negotiate per-diameter pricing (3.3mm, 4.0mm etc.). OEM packaging adds 8-12% cost. | $45 – $85/unit |
| Surgical Kit (Drill Guides, Drivers) | 30 kits | Request sterilization validation reports (ISO 11135) to avoid repackaging costs. | $180 – $320/kit |
| Prosthetic Components | 200 units | Insist on CAD/CAM compatibility files (STL, PLY) included at no extra cost. | $25 – $55/unit |
Negotiation Tip: Leverage 2026’s yuan depreciation (7.25 CNY/USD forecast) for 5-7% additional savings on FOB pricing. Always specify “ex-factory” pricing excludes export documentation fees (typically $150-300).
Step 3: Shipping & Logistics (DDP vs FOB 2026)
With 2026’s port congestion surcharges (Shanghai up 18% YoY), Incoterms selection is critical:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (avg. $3,200/40ft HC to Rotterdam). Hidden cost: Chinese export declaration fees ($220) |
Buyer bears cargo risk post-shipment. 2026 risk: 23% longer customs holds for medical devices |
Only for experienced importers with freight partners. Requires supplier to handle NMPA export docs |
| DDP (Delivered Duty Paid) | Supplier quotes all-inclusive price (FOB + freight + duties + VAT). 2026 avg. markup: 18-22% |
Supplier manages all risks until clinic/distributor warehouse. 2026 advantage: Covers new EU customs bond requirements |
Recommended for 90% of buyers. Verify “duties” include destination VAT (e.g., 20% UK VAT) |
2026 Critical Requirement: All shipments must include:
• EUDAMED-compliant UDI labels
• NMPA Export Certificate (出口医疗器械备案)
• Sterilization validation report (EN ISO 11135)
• Material traceability documentation (MTR)
Why Shanghai Carejoy is a Verified 2026 Sourcing Partner
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) meets all 2026 protocol requirements through:
- Regulatory Compliance: NMPA Class III License (国械注准20233170085) + EU MDR 2017/745 Certificate (NB: DE/9397) with full technical documentation available for audit
- Implant System Credentials: ISO 13485:2016 certified manufacturing (Scope: “Dental Implant Systems, Abutments, Surgical Kits”) with ASTM F136 mill test reports
- MOQ Flexibility: 30-set MOQ for All-on-4 protocol systems (2026 distributor tier pricing available)
- Logistics Expertise: DDP shipping to 47 countries with UDI-compliant labeling and EUDAMED registration support
- 19-Year Track Record: Zero regulatory rejections in EU/US/ANZ markets (2015-2025)
Note: Carejoy supplies complete implant systems (implants, abutments, surgical kits) alongside their core dental equipment portfolio.
Shanghai Carejoy Medical Co., LTD – 2026 Verified Partner
Factory Direct Sourcing Team
📍 Baoshan District, Shanghai, China (NMPA-Registered Facility)
✉️ [email protected]
💬 WhatsApp: +86 15951276160 (24/7 English Support)
🌐 www.carejoydental.com (Request 2026 Implant System Dossier)
Pro Tip: Reference “2026 ALL-ON-4 SOURCING GUIDE” for expedited regulatory documentation review.
1. Confirm supplier’s CE certificate includes “dental implant systems” under MDR (not legacy MDD)
2. Validate NMPA export备案 number via Chinese customs API
3. Require batch-specific Ra value reports for implant surfaces (target: 1.0-1.5 μm)
4. Audit sterilization validation (EO residual & SAL 10-6 proof)
5. Sign quality agreement specifying liability for regulatory non-compliance
© 2026 Global Dental Sourcing Consortium | This guide reflects regulatory standards as of January 2026.
All pricing based on Q4 2025 market analysis. Verify with suppliers for real-time quotes.
Disclaimer: All-on-4® is a registered trademark of Dentsply Sirona. This guide covers protocol-compatible systems.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: All-on-4® Dental Implant Systems
Target Audience: Dental Clinics & Medical Equipment Distributors – Updated for Q1 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements do All-on-4 surgical systems and compatible equipment have for global deployment in 2026? | All major All-on-4®-compatible surgical consoles (e.g., implant motors, CT-guided navigation units, and piezoelectric devices) are designed for global compatibility. Standard units support 100–240V AC, 50/60 Hz, making them suitable for use in North America, Europe, Asia, and Latin America. Dual-voltage models with auto-switching capability are now standard in 2026. Always verify the specific device label and include medical-grade surge protection and isolation transformers in high-voltage fluctuation regions. |
| 2. Are critical spare parts for All-on-4 surgical kits and prosthetic components readily available through authorized distributors in 2026? | Yes. As of 2026, all ISO 13485-certified All-on-4® system manufacturers maintain regional logistics hubs to ensure 72-hour delivery of critical spare parts, including torque drivers, healing caps, multi-unit abutments, surgical guides, and implant motors. Distributors are required to carry minimum inventory of high-wear components. We recommend clinics establish service contracts with OEMs or certified partners to guarantee access to genuine parts and avoid compatibility or warranty issues. |
| 3. What does the professional installation process for an All-on-4® treatment workflow entail, and is on-site technician support included? | Installation of a complete All-on-4® workflow includes integration of CBCT imaging, surgical planning software, guided surgery kits, and prosthetic delivery systems. In 2026, most premium system providers include on-site installation and calibration by certified biomedical engineers as part of the initial purchase or service agreement. This includes equipment setup, software configuration, staff training, and verification of torque accuracy and imaging alignment. Remote diagnostics and AI-assisted setup are now standard pre-installation steps. |
| 4. What is the standard warranty coverage for All-on-4® dental implants and associated surgical equipment purchased in 2026? | Implants themselves typically carry a lifetime warranty against fracture or failure when used per protocol. Surgical motors and navigation systems include a standard 3-year comprehensive warranty covering parts, labor, and software updates. Extended warranties (up to 5 years) are available through OEMs and include preventive maintenance and priority technical support. Warranty is contingent upon use of genuine components, scheduled servicing, and documented calibration records. |
| 5. Does the cost of All-on-4® systems in 2026 include installation, training, and ongoing technical support under warranty? | Entry-level system pricing typically covers hardware and software licenses only. However, by 2026, bundled “TotalCare” packages from leading manufacturers (e.g., Nobel Biocare, Straumann, Dentsply Sirona) include on-site installation, clinical training for surgical and prosthetic teams, 24/7 remote support, and first-year maintenance. These packages are increasingly standard for clinics purchasing full workflows. Distributors must clarify whether installation and training are included or offered as add-ons during procurement negotiations. |
Note: Pricing for All-on-4® implant systems varies by region, implant brand, prosthetic materials, and service inclusions. Refer to your authorized distributor for detailed quotations and compliance documentation.
Need a Quote for How Much Do All On 4 Dental Implants Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


