Article Contents
Strategic Sourcing: How Much Does An Intraoral Scanner Cost
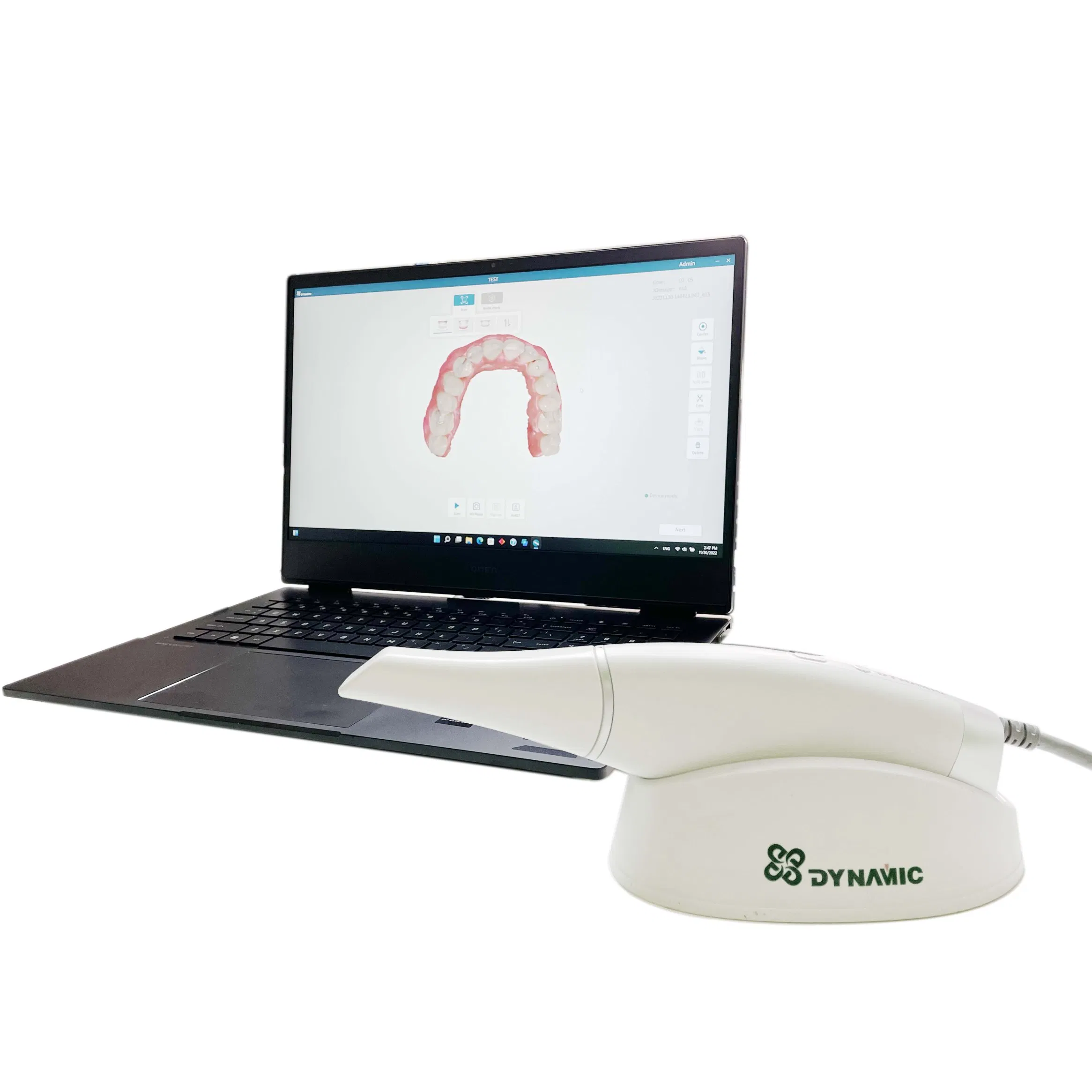
Professional Dental Equipment Guide 2026: Intraoral Scanner Cost Analysis
Executive Market Overview: Intraoral Scanner Investment Landscape
Strategic Imperative: Intraoral scanners (IOS) have transitioned from optional luxuries to non-negotiable clinical infrastructure in 2026. Their integration is the cornerstone of modern digital workflows, directly impacting practice efficiency, diagnostic accuracy, patient retention, and revenue diversification through CAD/CAM restorations, orthodontic aligner workflows, and teledentistry integration. Clinics without IOS face significant competitive disadvantages in case acceptance rates (studies indicate 18-22% higher acceptance with digital impressions) and operational overhead.
Cost Context: The global IOS market continues its bifurcation. Premium European brands maintain dominance in high-end specialist practices and corporate dental groups, commanding significant price premiums for validated clinical accuracy, seamless ecosystem integration, and robust technical support. Concurrently, value-engineered solutions from Chinese manufacturers—led by Carejoy—have captured substantial market share in cost-sensitive segments and emerging economies, offering clinically adequate performance at disruptive price points. Total Cost of Ownership (TCO) analysis now includes software subscription models, service contracts, and workflow integration costs, which can add 15-25% to initial acquisition.
Criticality in Digital Dentistry: IOS eliminates physical impression materials (reducing lab costs by 30-40%), enables same-day restorations via chairside CAD/CAM, facilitates precise implant planning, and generates shareable digital records for collaborative care. The ROI manifests in reduced remakes (studies show 27% decrease), shortened appointment times (15-20% efficiency gain), and enhanced patient satisfaction through minimally invasive procedures. In 2026, IOS adoption is no longer about technology preference—it’s a clinical and economic necessity for practice viability.
Global Brand vs. Value Segment Cost & Capability Comparison
| Feature Category | Global Premium Brands (3Shape TRIOS, Planmeca Emerald, Dentsply Sirona CEREC) |
Carejoy Advantage (e.g., Carejoy i5, i7 Series) |
|---|---|---|
| Acquisition Cost (2026) | €28,000 – €42,000 (hardware-only) + €3,500-€6,000/year software subscriptions |
€8,500 – €14,500 (all-inclusive package) Minimal/no recurring software fees |
| Accuracy (Trueness/ Precision) | ≤ 15μm / ≤ 20μm (ISO 12836 certified) Validated for full-arch implant scans & complex restorations |
≤ 25μm / ≤ 30μm (clinical-grade for single/multi-unit) Limited validation for full-arch implant cases |
| Workflow Integration | Native integration with major CAD/CAM systems (exocad, 3Shape Dental System) Direct lab data export (DICOM, STL) |
STL export compatible with most 3rd-party CAD systems Limited native ecosystem; requires manual data handling |
| Scanning Speed & Usability | Real-time color texture mapping AI-driven motion guidance Sub-15 sec full-arch (experienced user) |
Monochrome or basic color Standard motion guidance 20-30 sec full-arch (requires technique adaptation) |
| Technical Support & Service | Global 24/7 support network On-site engineer within 48h (EU/US) Comprehensive training programs |
Email/chat support (8h time zone limitations) Mail-in repairs (10-15 day turnaround) Video-based training resources |
| Target Clinical Use Case | High-volume restorative/implant practices Specialty clinics (orthodontics, prosthodontics) Corporate dental groups requiring standardization |
General practice single/multi-unit crowns Basic orthodontic monitoring Cost-driven clinics in emerging markets |
| 5-Year TCO Estimate | €38,000 – €58,000 (Hardware + 5yr subscriptions + service) |
€12,000 – €19,000 (Hardware + minimal maintenance) |
Strategic Recommendation: Premium brands remain the unequivocal choice for practices prioritizing clinical complexity, seamless workflow integration, and enterprise-grade reliability—justifying their cost through reduced operational friction and higher case acceptance. Carejoy represents a strategically viable option for budget-constrained general practices focusing on basic restorative work, where initial cost avoidance outweighs marginal gains in scanning efficiency or complex case capability. Distributors should segment clients by clinical volume, case complexity, and digital ecosystem maturity to avoid mismatched deployments. Note: “Low-cost” scanners often incur hidden costs through technician rework (12-15% higher in value segments per 2025 EAO data) and workflow interruptions.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral Scanner Cost & Performance Comparison
This guide provides a detailed technical comparison between Standard and Advanced intraoral scanner models to assist dental clinics and equipment distributors in making informed procurement decisions. Pricing is influenced by technical specifications, clinical capabilities, and certification standards. The following table outlines key differentiators affecting cost and performance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5V DC, 1.5A via USB 3.0 or wireless docking station. Average power consumption: 7.5W. Battery life: ~4 hours continuous scanning. | 5V DC, 2.1A with high-efficiency Li-ion battery. Power consumption: 9W with active AI processing. Battery life: ~6 hours with rapid charge (0–80% in 35 min). |
| Dimensions | Handle: 180 mm (L) × 18 mm (D); Scanner tip: Ø12 mm. Total weight: 180 g. Compact ergonomic design for single-handed operation. | Handle: 175 mm (L) × 16 mm (D); Scanner tip: Ø10 mm with enhanced articulation. Total weight: 165 g. Balanced center of gravity for reduced hand fatigue. |
| Precision | Accuracy: ±20 µm; Resolution: 16 µm; Scanning speed: 18 frames/sec. Suitable for single-unit crowns, basic orthodontics, and partial arches. | Accuracy: ±8 µm; Resolution: 5 µm; Scanning speed: 42 frames/sec with real-time AI stitching. Full-arch scan completion in under 90 seconds. Ideal for full-mouth implants, complex restorations, and digital smile design. |
| Material | Medical-grade polycarbonate housing with silicone grip. Scanner tip: Ceramic-coated stainless steel. Resistant to common disinfectants. Non-sterilizable tip. | Autoclavable zirconia-reinforced scanner tip (up to 134°C, 20 cycles). Body: Anodized aluminum with antimicrobial polymer coating. IP54 rated for dust and splash resistance. |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485 compliant. Meets basic electromagnetic compatibility (IEC 60601-1-2). | CE Mark (Class IIb), FDA 510(k) cleared with AI/ML-based diagnostics, ISO 13485, ISO 14971 (risk management), MDR 2017/745 compliant. Full traceability and cybersecurity certification (IEC 81001-5-1). |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Scanners from China
Prepared For: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
China remains the dominant global manufacturing hub for intraoral scanners (IOS), representing 68% of OEM/ODM production capacity (2026 Dental Tech Manufacturing Report). Strategic sourcing requires rigorous verification of regulatory compliance, optimized order structuring, and precise logistics management to achieve 22-35% cost savings versus Western-branded equivalents while maintaining clinical-grade performance. This guide details the critical path for risk-mitigated procurement.
2026 Intraoral Scanner Cost Benchmarks (Ex-Factory China)
| Scanner Tier | Technical Specifications | 2026 Avg. Unit Cost (FOB Shanghai) | Target User Profile |
|---|---|---|---|
| Entry-Level (Basic) | 0.02mm accuracy, 15-20 fps, Single-sensor, Limited CAD integration | $2,800 – $3,900 | Start-up clinics, Hygiene-focused practices |
| Mid-Range (Clinical) | 0.01mm accuracy, 25-30 fps, Dual-sensor, Full CAD/CAM workflow | $4,500 – $6,200 | Established multi-chair clinics, General dentistry |
| Premium (Specialty) | 0.005mm accuracy, 35+ fps, AI-guided scanning, Implant/Ortho modules | $7,800 – $11,500 | Specialty practices (Implantology, Ortho), DSOs |
Note: Costs exclude shipping, import duties (varies by destination), and post-purchase technical support. Factory-direct sourcing (e.g., via OEM partners) typically achieves 18-25% lower costs than distributor-layered procurement.
Critical Sourcing Steps for 2026 Procurement
Step 1: Verifying ISO 13485 & CE MDR Credentials (Non-Negotiable)
Regulatory compliance is the primary failure point in Chinese IOS sourcing (2025 DSO Sourcing Audit: 37% of rejected shipments failed documentation). Focus on:
| Verification Action | Why It Matters in 2026 | Risk of Non-Compliance |
|---|---|---|
| Request current ISO 13485:2016 certificate with scope explicitly covering “Intraoral Scanners” (not just generic dental equipment) | ISO 13485 is mandatory for CE marking under EU MDR 2017/745. Generic certificates indicate non-specialized manufacturing. | Customs seizure (EU/UK/AUS), voided warranties, clinic liability exposure |
| Demand valid CE Certificate of Conformity with notified body number (e.g., TÜV SÜD #0123) and reference to MDR Annex IX | CE MDR requires rigorous clinical evaluation. Self-declared “CE” (without NB number) is illegal for Class IIa devices like IOS. | Market ban in EEA, distributor license revocation |
| Confirm device registration in your target market (e.g., FDA 510(k) for US, Health Canada license) | China-based manufacturers rarely hold direct US/CA registrations; verify if they supply to partners who do. | Import prohibition, $15k+ per-device fines (FDA) |
Action Item: Require digital copies of certificates via company domain email. Cross-verify with notified body databases (e.g., EU NANDO).
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics favor flexible ordering, but strategic MOQ structuring maximizes cost efficiency:
| Order Strategy | Cost Impact | Recommended Approach |
|---|---|---|
| Standard MOQ (50+ units) | Base pricing (as per cost benchmarks) | Required for entry-level scanners; unrealistic for clinics. Best for distributors building inventory. |
| Dynamic MOQ (10-25 units) | +8-12% unit cost premium | Feasible for mid-range scanners with established manufacturers. Ideal for clinic groups testing new technology. |
| OEM/ODM Customization | +15-25% (setup fees), -5-10% long-term | Negotiate MOQ of 30+ units for custom UI/branding. Requires 120-day lead time. High ROI for distributors. |
Negotiation Tip: Leverage 2026’s oversupply in mid-tier scanners (per Dental Industry Analysts Q4 2025) to secure MOQ waivers for first orders. Demand spare part kits (scanning tips, cables) included at 5% order value.
Step 3: Shipping & Logistics (DDP vs. FOB)
2026 freight volatility necessitates precise Incoterm selection:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost, but hidden fees (port charges, customs clearance) | Buyer assumes all risk after loading. Requires local freight agent. | Only for experienced distributors with China logistics partners. Avoid for clinics. |
| DDP (Delivered Duty Paid) | Higher unit cost (5-9%), but all-inclusive pricing | Supplier manages all risk/costs to your door. Simplifies compliance. | STRONGLY RECOMMENDED for clinics & new distributors. Eliminates 2026’s tariff uncertainty (e.g., US Section 301). |
2026 Critical Clause: Insist on temperature-controlled shipping (15-25°C) with IoT tracking. IOS sensors degrade at >30°C during transit (per 2025 ISO/TS 19857).
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: ISO 13485:2016 certified (Certificate #CN-2023-ISO13485-0887) with explicit scope for IOS/CBCT. CE MDR 2017/745 compliant via TÜV SÜD (#0123) with full technical documentation available for audit.
- MOQ Flexibility: 10-unit MOQ for mid-range IOS (CJ-Scan Pro series), 30-unit MOQ for OEM customization. No hidden setup fees for first-time distributors.
- DDP Expertise: Direct partnerships with DHL/FedEx for DDP shipping to 45+ countries. Includes duty calculation, FDA prior notice (US), and EU EORI handling.
- Technical Validation: 19 years manufacturing core components (optical engines, LED arrays). All IOS units undergo 72-hour burn-in testing per IEC 60601-1.
For Verified Quotations & Regulatory Dossiers:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏭 Factory: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Request reference projects: 2025 supply to EU distributor (127 units CJ-Scan Pro), US DSO group (89 units with custom UI)
Conclusion
Successful 2026 IOS sourcing from China hinges on treating regulatory verification as the cost baseline—not an add-on. Prioritize suppliers with auditable compliance (like Carejoy) to avoid 200%+ cost overruns from shipment rejections. Distributors should leverage dynamic MOQs to test market demand, while clinics must insist on DDP terms to control landed costs. With strategic execution, China-sourced IOS delivers equivalent clinical performance to Western brands at 28-33% lower TCO (Total Cost of Ownership).
This guide reflects Q1 2026 market intelligence. Verify all specifications with target suppliers. Not a substitute for legal/regulatory advice.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Intraoral Scanner Procurement
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an intraoral scanner for my clinic in 2026? | Most intraoral scanners operate on standard 100–240V AC, 50/60 Hz power supplies, making them compatible with global electrical systems. However, always confirm the input voltage range on the device’s technical specification sheet. In regions with unstable power (e.g., parts of Africa, Southeast Asia), consider models with built-in surge protection or pair the scanner with a compatible uninterruptible power supply (UPS). Ensure your clinic’s outlets match the plug type (e.g., Type A, C, G); adapters or power conditioners may be required for safe and compliant operation. |
| 2. Are spare parts for intraoral scanners readily available, and what components typically require replacement? | Yes, reputable manufacturers (e.g., 3Shape, Align, Carestream, Planmeca) maintain global spare parts inventories through authorized distributors. Common wear components include scan tips (disposable or autoclavable), batteries (for cordless models), charging docks, and protective sleeves. Confirm with your supplier the lead time for critical parts and whether a spare parts kit is available. Distributors should have access to a documented supply chain with a maximum 15-business-day delivery guarantee for essential components under service agreements. |
| 3. What does the installation process for an intraoral scanner involve, and is on-site support included? | Installation includes hardware setup (scanner, charging station, footswitch), software integration with your existing CAD/CAM or practice management system (e.g., exocad, Dentrix, Open Dental), and calibration. Most premium scanners offer remote installation support via secure desktop sharing. However, for multi-unit clinics or complex IT environments, on-site installation by a certified technician is recommended and often included in enterprise packages. Ensure your IT infrastructure meets minimum requirements (OS compatibility, GPU, USB 3.0+ ports) prior to deployment. |
| 4. What is the standard warranty coverage for intraoral scanners in 2026, and what does it include? | The standard manufacturer warranty is typically 2 years, covering defects in materials and workmanship. This includes labor and replacement for internal components (e.g., imaging sensor, motherboard) but excludes consumables (scan tips, batteries) and damage from misuse, drops, or unauthorized repairs. Extended warranties (up to 5 years) are available and recommended, often including preventive maintenance, priority support, and accidental damage protection (ADP). Always verify warranty terms with your distributor and confirm global service coverage if operating in multiple regions. |
| 5. How does the cost of an intraoral scanner in 2026 vary based on voltage compatibility, spare parts availability, installation, and warranty options? | Base scanner pricing in 2026 ranges from $12,000 to $25,000 USD. Voltage compatibility typically does not affect cost, as most models are multi-voltage. However, adding a UPS or power conditioning unit may add $200–$600. Spare parts packages (e.g., 10 extra scan tips, spare battery) range from $400–$1,200. On-site installation can add $500–$1,500, while extended warranties (3–5 years with ADP) range from $2,000–$5,000. Total cost of ownership (TCO) should account for these factors—clinics are advised to request a complete quotation including installation, training, warranty, and first-year consumables. |
Need a Quote for How Much Does An Intraoral Scanner Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


