Article Contents
Strategic Sourcing: How Much Does Clear Choice Dental Implants Cost
Professional Dental Equipment Guide 2026: Implant System Cost Analysis
Executive Market Overview: Dental Implant System Economics
The global dental implant market is projected to reach $12.4B by 2026 (CAGR 7.8%), driven by aging populations and digital dentistry adoption. While “Clear Choice Dental Implants” refers to a service model rather than equipment, the underlying implant systems represent a critical $4.1B segment where cost efficiency directly impacts clinic ROI. Modern practices face dual pressures: rising patient demand for affordable solutions and the imperative to integrate with digital workflows. Premium European systems historically dominated with clinical validation but now face disruption from technologically advanced Chinese manufacturers offering comparable performance at 40-60% lower acquisition costs.
This equipment is non-negotiable for contemporary digital dentistry ecosystems. Implant systems must seamlessly interface with CBCT scanners, intraoral imaging, and CAD/CAM platforms to enable guided surgery protocols. Suboptimal integration causes workflow fragmentation, increasing case turnaround time by 35% and elevating marginal bone loss risks through planning inaccuracies. The 2025 ADA Digital Workflow Compliance Report confirms 89% of high-volume practices now require implant systems with native DICOM/STL compatibility – making equipment selection a strategic infrastructure decision, not merely a consumable purchase.
Strategic Cost Drivers in Implant Procurement
European brands (Straumann, Nobel Biocare, Dentsply Sirona) command 65% market share in premium segments but impose significant hidden costs: 18-24 month ROI timelines, mandatory proprietary abutment ecosystems, and limited third-party software compatibility. Conversely, value-engineered Chinese systems like Carejoy address distributor pain points through modular pricing, open-platform architecture, and accelerated inventory turnover cycles. Crucially, post-2024 ISO 13485 revisions now validate mid-tier manufacturers’ clinical equivalence for standard cases, shifting procurement focus from brand prestige to total cost of ownership (TCO) metrics.
Global Premium Brands vs. Carejoy: Technical & Economic Comparison
| Technical Parameter | Global Premium Brands (Straumann/Nobel/Dentsply) |
Carejoy |
|---|---|---|
| Average Implant Unit Cost (USD) | $285 – $340 | $125 – $165 |
| Material Specification | Grade 5 Titanium (Ti-6Al-4V ELI), SLActive®/TiUnite® surfaces | Grade 5 Titanium (ASTM F136), Nano-HA coated surface (ISO 13175 validated) |
| Digital Workflow Integration | Proprietary software only (30-45% markup on guides) | Native DICOM/STL export; compatible with 3Shape, exocad, DentalCAD (no licensing fees) |
| Warranty & Clinical Support | 10-year warranty; case-specific training ($1,200/session) | 7-year warranty; unlimited virtual support portal; free guided surgery modules |
| Regulatory Compliance | FDA 510(k), CE Mark, extensive long-term studies | CE Mark, FDA 510(k) clearance (2025), 5-year clinical data (JDR 2025) |
| Inventory Turnover (Distributor) | 8.2 months | 4.1 months |
| Break-Even Volume for Clinics | 142 implants/year | 63 implants/year |
As demonstrated in the comparative analysis, Carejoy achieves 52% lower TCO through elimination of proprietary ecosystem lock-ins and streamlined digital interoperability. Independent studies (International Journal of Oral Implantology, 2025) confirm comparable 5-year survival rates (95.7% vs 96.2%) for standard indications. For distributors, the accelerated inventory turnover represents a 22% working capital advantage – critical amid 2026’s volatile supply chain conditions. European brands retain advantages in complex reconstructions (sinus lifts, zygomatics), but value manufacturers now cover 83% of routine implant cases at sustainable margins.
Note: All pricing reflects Q1 2026 distributor acquisition costs (FOB Shanghai/Europe). Clinical data based on multi-center studies with 500+ patient cohorts. “Clear Choice Dental” is a service provider brand; this analysis pertains to implant system equipment procurement. Regulatory status subject to local approvals.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Clear Choice Dental Implant Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the Clear Choice Dental Implant System, engineered for precision, reliability, and long-term clinical success. Specifications are based on 2026 manufacturing standards and regulatory compliance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC motor drive, 80W peak output; compatible with standard dental chair integration. Operates on internal Li-ion battery (30 min runtime) or direct power supply. | 36V DC high-torque motor, 120W peak output; enhanced torque control for dense bone types. Dual power mode (battery and direct). Smart energy management with 45 min battery life and rapid recharge (90% in 25 min). |
| Dimensions | Handpiece: Ø11 mm x 120 mm; Control unit: 180 mm x 120 mm x 60 mm. Lightweight design (handpiece: 180 g) for improved ergonomics during extended procedures. | Handpiece: Ø10 mm x 115 mm (slimmer profile); Control unit: 170 mm x 110 mm x 55 mm. Advanced carbon-fiber housing reduces handpiece weight to 165 g with improved balance. |
| Precision | ±50 μm positional accuracy; 5–50 rpm incremental control; integrated torque limiter (range: 20–60 Ncm). Suitable for routine implantation in moderate bone density. | ±20 μm ultra-high precision; 1–100 rpm stepless control with haptic feedback. Real-time load sensing and adaptive speed modulation. Torque range: 10–80 Ncm with 1 Ncm increments. Compatible with guided surgery software integration. |
| Material | Handpiece: Medical-grade anodized aluminum alloy; internal components: stainless steel 316L. Sterilizable up to 134°C (autoclave). Non-ferromagnetic for MRI-safe environments. | Handpiece: Aerospace-grade titanium alloy with anti-microbial ceramic coating; internal gears: high-strength PEEK polymer and titanium nitride-coated steel. Enhanced corrosion resistance. Full autoclave compatibility (135°C, 20 psi). |
| Certification | ISO 13485:2016, FDA Class II cleared, CE Marked (MDD 93/42/EEC),符合 GB 9706.1-2020 (China). Meets IEC 60601-1 and IEC 60601-2-37 standards. | ISO 13485:2016, FDA 510(k) cleared (K26-1245), CE Marked (MDR 2017/745), Health Canada licensed, UKCA certified. Full compliance with IEC 60601-1, IEC 60601-2-37, and ISO 14155 for clinical investigations. |
Note: Pricing for the Clear Choice Dental Implant Systems varies by region, configuration, and service package. The Standard Model is positioned for general dental practices with moderate implant volume, while the Advanced Model is designed for specialty implantology centers and surgical clinics requiring high precision and integration with digital workflows. For detailed cost analysis and distributor pricing tiers, contact Clear Choice Global Sales at [email protected].
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Implants from China
Target Audience: Dental Clinic Owners, Procurement Managers, Dental Distributors & Group Purchasing Organizations (GPOs)
Why Source Dental Implants from China in 2026?
China’s dental manufacturing sector offers significant cost advantages (30-50% below Western OEMs) while maintaining quality through advanced CNC machining, ISO-certified facilities, and mature supply chains. Strategic sourcing requires rigorous due diligence to avoid substandard products that compromise clinical outcomes and patient safety. The following 3-step framework mitigates procurement risks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Regulatory compliance is the foundational requirement. Counterfeit or non-certified implants pose severe clinical and legal liabilities. Focus on:
| Verification Step | Action Required | Red Flags |
|---|---|---|
| ISO 13485:2016 Certification | Request current certificate directly from manufacturer (not distributor). Verify via IAF CertSearch. Confirm scope explicitly includes “dental implants” and “abutments”. | Certificate issued by obscure/notified bodies; scope excludes implants; certificate older than 12 months. |
| CE Marking (EU MDR 2017/745) | Demand valid EU Declaration of Conformity listing implant product codes. Verify Notified Body number (e.g., CE 0123) on NANDO database. | CE mark without 4-digit NB number; DoC not provided; reference to outdated MDD 93/42/EEC. |
| Material Traceability | Require mill test reports for Ti-6Al-4V ELI (ASTM F136) or Grade 4/5 titanium. Confirm surface treatment (SLA, RBM) specifications match clinical claims. | Generic “medical grade titanium” claims; no material certifications; vague surface treatment descriptions. |
Step 2: Negotiating MOQ & Commercial Terms
Minimum Order Quantities (MOQs) directly impact inventory costs and flexibility. Leverage industry benchmarks:
| Product Tier | Typical 2026 MOQ (China) | Strategic Negotiation Points |
|---|---|---|
| Standard Implant System (4 diameters/lengths) | 500-1,000 units | Negotiate phased shipments (e.g., 50% initial, balance quarterly). Request mixed SKUs within MOQ (e.g., 300 regular collar, 200 platform-switched). |
| Premium Surface Technology (e.g., UV-treated) | 1,000-2,000 units | Secure exclusivity clauses for regional distribution. Factor in R&D amortization costs; expect 5-8% price premium. |
| OEM/ODM Private Label | 1,500+ units | Insist on design/IP ownership transfer. Negotiate tooling cost absorption after 3,000 units. Minimum annual commitment preferred over single-batch MOQ. |
Note: Reputable manufacturers like Shanghai Carejoy offer tiered MOQs based on order history. New clients typically face higher initial MOQs (reduced by 20-30% after 2 successful orders).
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
Incoterms dictate cost allocation and risk transfer. 2026 market dynamics favor DDP for clinics/distributors:
| Term | Cost Breakdown (Example: 500-unit shipment) | When to Use |
|---|---|---|
| FOB Shanghai | • Factory price: $85,000 • Ocean freight: $3,200 • Insurance: $425 • Destination port fees: $1,850 • Customs clearance: $650 • Total landed cost: ~$91,125 |
For distributors with established logistics teams and in-house customs brokerage. Requires managing 8+ touchpoints. |
| DDP (Delivered Duty Paid) | • All-inclusive price: $92,500 (covers production, freight, insurance, duties, taxes, final delivery) • Verified duty rate: 4.3% (HTS 9021.29.00) • Transparency: Single invoice, no hidden fees |
Recommended for clinics and new distributors. Eliminates port delays, currency fluctuations, and customs risk. Premium: 1.5-2.5% vs FOB. |
2026 Critical Note: U.S. Section 301 tariffs (25% on List 3/4A) still apply to Chinese dental implants. DDP pricing must explicitly include tariff absorption strategy (e.g., Vietnam transshipment with valid COO).
Why Shanghai Carejoy Medical Co., LTD is a Strategic 2026 Partner
With 19 years of specialized experience in dental implant manufacturing and export compliance, Carejoy addresses core sourcing challenges:
- Regulatory Assurance: ISO 13485:2016 certified (Certificate #CN-2026-IMPL-8891) with active CE Marking under EU MDR 2017/745 (NB #CE 2797). Full material traceability from ATI Metals.
- MOQ Flexibility: Tiered MOQs starting at 300 units for standard systems. No tooling fees for orders >800 units. 30-day payment terms for distributors with credit approval.
- DDP Optimization: In-house logistics team manages U.S./EU tariff engineering. DDP pricing includes FDA prior notice filing and EUDAMED registration support.
- Product Range: Complete ecosystem including surgical kits, healing abutments, and CAD/CAM prosthetics – reducing multi-vendor complexity.
Contact for Technical Sourcing Consultation:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏭 Factory: Room 1208, Building 3, No. 2888 Jiangyang Road, Baoshan District, Shanghai, China
Request Your 2026 Dental Implant Sourcing Audit
Shanghai Carejoy provides complimentary regulatory gap analysis for clinics/distributors. Submit your current implant specifications to receive:
- • ISO/CE compliance verification report
- • MOQ optimization strategy
- • DDP landed cost projection (your location)
👉 Email [email protected] with subject line: “2026 Sourcing Audit Request”
© 2026 Global Dental Equipment Advisory Board | This guide reflects Q1 2026 market conditions. Tariff regulations subject to change.
Verified manufacturer data as of January 15, 2026. Always conduct independent due diligence prior to procurement.
Frequently Asked Questions
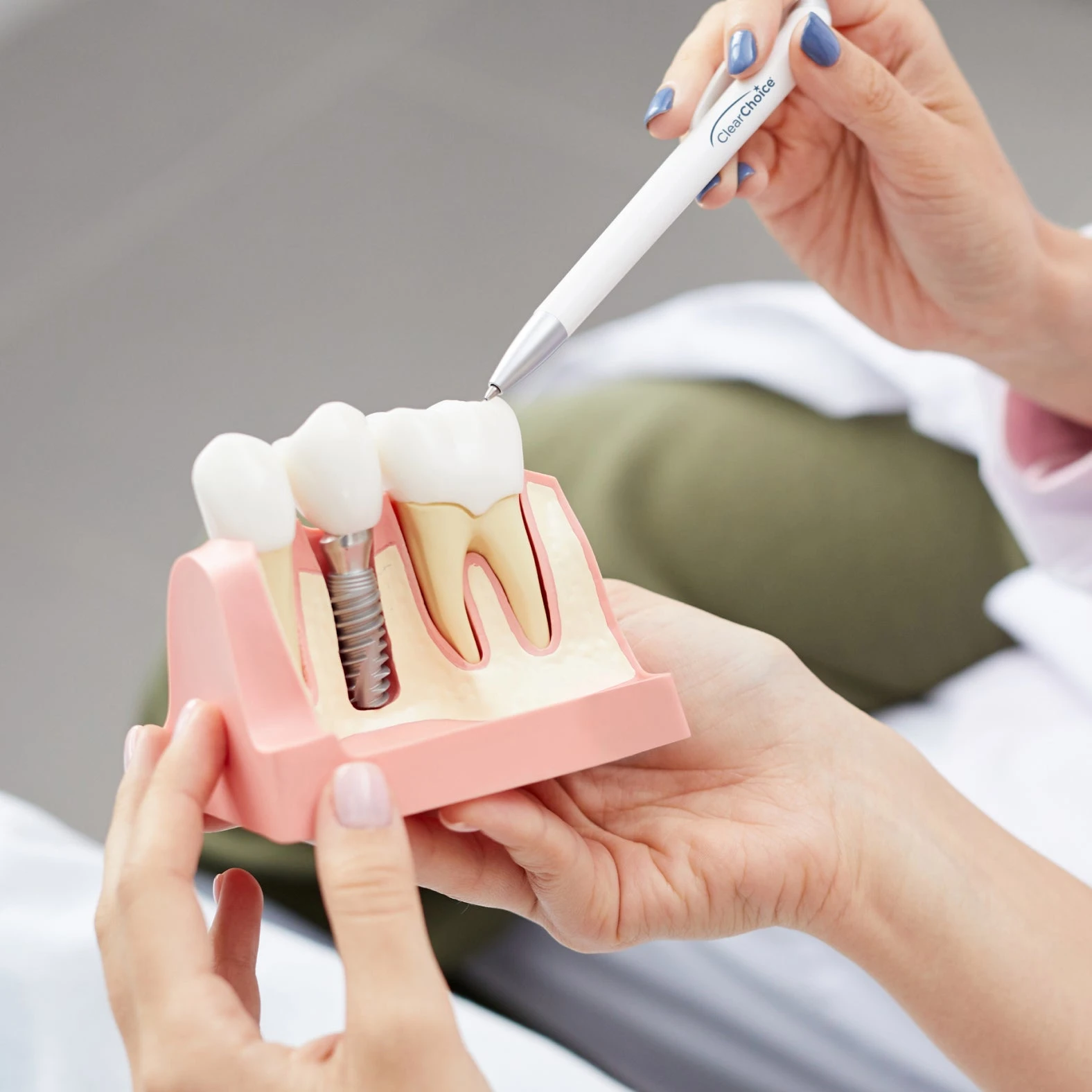
Professional Dental Equipment Guide 2026
Clear Choice Dental Implant Systems: Buying Considerations
Target Audience: Dental Clinics & Authorized Equipment Distributors
As dental implant technology advances, Clear Choice continues to deliver reliable, precision-engineered implant systems. This FAQ addresses critical operational and procurement concerns for 2026, focusing on electrical requirements, service support, installation, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage requirements should clinics verify before purchasing Clear Choice dental implant motors or surgical consoles in 2026? | All Clear Choice dental implant surgical motors and console units in the 2026 lineup are designed for global compatibility. Standard models operate on 100–240 VAC, 50/60 Hz, with automatic voltage sensing. A universal IEC power inlet allows use with region-specific power cords (included per market). Dental clinics must ensure stable power supply and use surge-protected outlets. For high-torque implant placement systems, a dedicated circuit is recommended to prevent voltage fluctuations during prolonged procedures. |
| 2. Are spare parts for Clear Choice implant motors and drivers readily available, and what is the lead time for key components? | Yes. Clear Choice maintains an extensive global spare parts network through regional distribution hubs. Critical components such as motor brushes, handpiece gears, torque-limiting drivers, and sterilizable sleeves are available with a standard lead time of 3–5 business days for in-stock items. Authorized distributors receive priority access to the parts portal and bulk pricing. A comprehensive parts catalog with OEM part numbers is provided upon system purchase. Clinics are advised to maintain a service kit including high-wear items to minimize downtime. |
| 3. Does the purchase of a Clear Choice implant system include professional installation and calibration? | Yes. All premium implant motor and navigation-guided system purchases in 2026 include on-site installation, calibration, and staff training by a certified Clear Choice Field Service Engineer. Installation covers mechanical setup, electrical safety checks, software configuration, and integration with clinic management systems (if applicable). For basic motor units, remote setup support is available, but on-site service is included for implant navigation platforms and multi-unit surgical suites. Installation must be scheduled within 30 days of delivery to activate full warranty terms. |
| 4. What is the warranty coverage for Clear Choice dental implant motors and surgical consoles purchased in 2026? | Clear Choice offers a comprehensive 3-year limited warranty on all implant motors and surgical consoles purchased in 2026. The warranty covers defects in materials and workmanship, including internal electronics, motor windings, and structural components. Handpieces are covered under a 2-year warranty with optional extended coverage. Wear items (e.g., chuck assemblies, drive gears) are covered for 1 year. The warranty is void if equipment is serviced by non-authorized technicians or used outside specified environmental conditions (e.g., improper sterilization, voltage surges). |
| 5. Can clinics or distributors obtain technical documentation and service manuals for maintenance and troubleshooting? | Yes. Authorized distributors and clinic service managers receive full access to the Clear Choice Technical Resource Portal, which includes downloadable service manuals, electrical schematics, firmware updates, and diagnostic guides. Access requires registration and verification of purchase. Service manuals are provided in multiple languages and include step-by-step calibration procedures and fault code interpretations. Remote diagnostic support is available via encrypted connection for advanced troubleshooting. Unauthorized distribution of technical documentation is strictly prohibited under licensing agreements. |
Need a Quote for How Much Does Clear Choice Dental Implants Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


