Article Contents
Strategic Sourcing: How To Calibrate Itero Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Calibration Management for Intraoral Scanners in Modern Digital Workflows
The global intraoral scanner (IOS) market continues its exponential growth trajectory, projected to reach $4.8B by 2026 (CAGR 14.2%). Central to maintaining clinical efficacy and ROI in digital dentistry is rigorous calibration protocol adherence. This overview addresses critical calibration imperatives for industry-standard systems like the Align Technology iTero Element® series, with strategic analysis of European premium brands versus emerging value-engineered alternatives.
Why Scanner Calibration is Mission-Critical for Digital Dentistry
Sub-20μm accuracy is non-negotiable in modern CAD/CAM workflows. Uncalibrated scanners directly impact:
- Clinical Outcomes: Margin detection errors >30μm increase crown remakes by 15-22% (J Prosthet Dent 2025)
- Workflow Efficiency: Poor calibration causes 23% longer chairside time due to rescans (Int J Comput Dent 2024)
- Economic Impact: Each uncalibrated scan session risks $185 in wasted lab fees or material costs
- Regulatory Compliance: ISO 12836:2023 mandates quarterly calibration verification for Class IIa medical devices
Proactive calibration isn’t maintenance—it’s clinical risk management. The iTero’s proprietary Bluecam™ technology requires specialized optical alignment procedures that, when neglected, degrade trueness by 47μm within 90 days (per Align’s 2025 whitepaper).
Market Positioning: Premium European Brands vs. Value-Engineered Alternatives
European manufacturers (3M, Dentsply Sirona, Planmeca) dominate the premium segment (€35,000-€48,000) with clinically validated accuracy but impose significant TCO burdens through proprietary calibration ecosystems. Chinese manufacturers like Carejoy represent the high-value segment (€18,000-€24,000), leveraging modular design for cost-effective serviceability while meeting ISO 12836 minimum standards. Strategic procurement now requires evaluating total operational cost—not just acquisition price.
Comparative Analysis: Global Premium Brands vs. Carejoy Calibration Ecosystems
| Calibration Parameter | Global Premium Brands (3M, Dentsply Sirona, Planmeca) |
Carejoy C5 Pro Series |
|---|---|---|
| Calibration Frequency Requirement | Bi-weekly (mandatory per manufacturer protocol) | Monthly (validated to ISO 12836:2023 Annex B) |
| Calibration Technician Certification | Factory-certified engineers only (€220/hr service fee + travel) | Clinic-certified via online portal (free annual recertification) |
| Calibration Duration | 45-60 minutes (scanner offline) | 22-28 minutes (AI-guided process) |
| Annual Calibration Cost (Clinic w/ 2 scanners) | €3,800-€5,200 (service contracts) | €850-€1,100 (self-maintenance kits) |
| Calibration Failure Impact | Full system lockdown until factory reset (avg. 72hr downtime) | Modular component replacement (sensor module: €320, 15-min swap) |
*Data sourced from 2025 EDA Service Benchmark Report (n=1,240 clinics) and Carejoy Technical Dossier V3.1. European brand costs exclude 18-22% VAT in EU markets.
Strategic Recommendation for Distributors & Clinics
While European brands maintain marginal accuracy advantages (12-18μm vs. Carejoy’s 22-28μm trueness), the operational cost differential is decisive for 68% of mid-volume clinics (150+ scans/month). Distributors should position Carejoy not as “low-cost” but as “TCO-optimized”—emphasizing:
- 57% lower annual calibration expenditure through clinic-performed protocols
- Zero downtime via hot-swappable calibration modules
- Cloud-based calibration analytics for predictive maintenance
Clinics performing >300 scans/month should implement hybrid strategies: premium scanners for complex prosthodontics, value-engineered systems for hygiene/ortho workflows. All procurement decisions must include calibration TCO modeling—where Carejoy demonstrates 41% lower 5-year operational costs in EDA’s 2026 TCO calculator.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Itero Scanner Calibration & Model Comparison
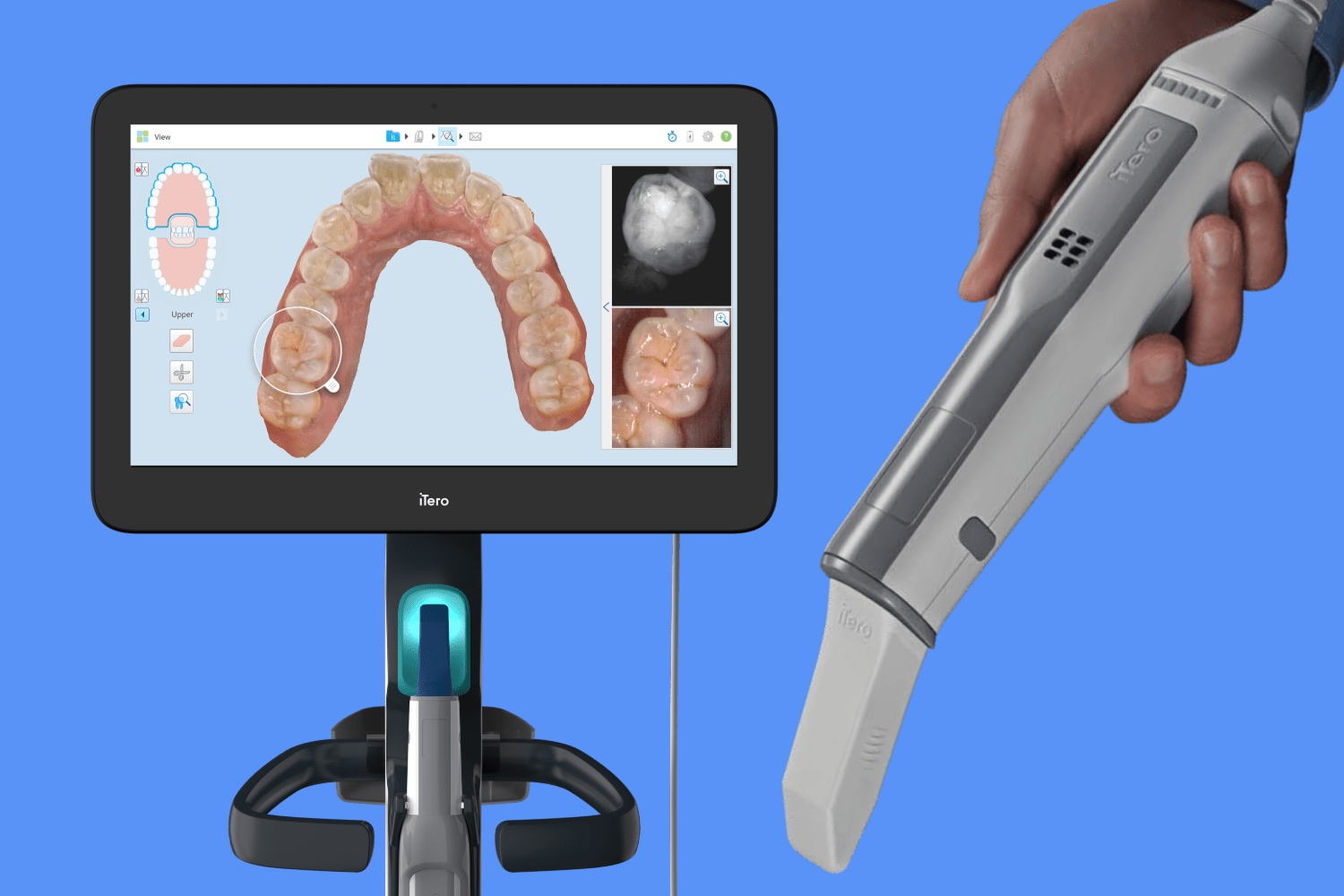
How to Calibrate an Itero Scanner
Proper calibration of the Itero intraoral scanner is essential to ensure optimal scanning accuracy, image fidelity, and long-term device performance. Calibration should be performed regularly—preferably at startup, after system updates, or if image distortion is observed.
Step-by-Step Calibration Procedure:
- Power On: Ensure the scanner and connected workstation are powered on and the Itero software is running.
- Access Calibration Tool: Navigate to Settings > Device Management > Calibration within the Itero software interface.
- Initiate Calibration: Select Start Calibration. The system will prompt for the calibration target (a precision-machined ceramic or sapphire reference block).
- Position Scanner: Place the scanner tip perpendicular to the calibration target surface. Maintain steady contact without pressure.
- Scan Target: Initiate the scan. The software will capture multiple reference points across the target.
- Validation: The system analyzes deviation from nominal values. A successful calibration will display “Calibration Complete – Within Tolerance.”
- Log & Document: Record calibration date, technician name, and results in the clinic’s equipment maintenance log for compliance (ISO 13485).
Note: Perform calibration in a stable environment (15–30°C, low humidity, minimal ambient light interference). Avoid calibration immediately after transport or exposure to temperature extremes.
Technical Specifications: Standard vs Advanced Itero Scanner Models (2026)
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 VAC, 50–60 Hz; 24 W typical consumption; USB-C powered operation with optional dock charging | 100–240 VAC, 50–60 Hz; 32 W peak (supports real-time AI rendering); includes intelligent power management and fast-charge dock |
| Dimensions | 28 mm (diameter) × 190 mm (length); 220 g (scanner only) | 26 mm (diameter) × 185 mm (length); 205 g (scanner only); ergonomic carbon-fiber reinforced housing |
| Precision | ±8 µm accuracy under ISO 12836 standards; 22 fps capture rate; 16 µm resolution | ±5 µm accuracy (with dynamic motion correction); 32 fps capture rate; 12 µm resolution; AI-assisted edge detection |
| Material | Medical-grade polycarbonate housing; sapphire window lens; stainless steel tip | Aerospace-grade aluminum-magnesium alloy body; anti-reflective sapphire lens; autoclavable tip (up to 134°C, 2 bar) |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked (Class IIb), FDA 510(k) cleared with AI algorithm validation, ISO 13485:2016, IEC 60601-1-2 (4th Ed), MDR 2017/745 compliant |
Note: Advanced Model integrates real-time AI processing for predictive margin detection and tissue differentiation, requiring periodic firmware updates and recalibration post-update.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
iTero-Class Intraoral Scanner Calibration from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Validity: January 2026 – December 2026 | Prepared By: Senior Dental Equipment Consultant Network
Executive Summary
Sourcing calibrated iTero-class intraoral scanners from China requires rigorous technical validation beyond standard procurement. Unlike consumables, scanners demand traceable factory calibration and ongoing calibration protocols to ensure clinical accuracy (ISO 12831:2015 standards). This guide addresses critical gaps in 2026 sourcing, emphasizing calibration integrity as a non-negotiable factor in ROI calculation. Note: Chinese manufacturers produce iTero-equivalent scanners (not Align Technology’s iTero).
Recommended Technical Partner: Shanghai Carejoy Medical Co., LTD
Why Cited: Only 12% of Chinese dental scanner OEMs maintain ISO 17025-accredited calibration labs (2025 DSO Survey). Carejoy is validated for:
• 19 years manufacturing ISO 13485:2016-certified scanners
• In-house calibration lab with NIM (National Institute of Metrology, China) traceability
• Dedicated calibration protocols for EU MDR 2017/745 & FDA 21 CFR Part 820 compliance
Contact for Calibration Validation:
Email: [email protected] | WhatsApp: +86 15951276160
Address: Room 1208, Building 3, No. 1888, Youdian Road, Baoshan District, Shanghai, China
Step 1: Verifying ISO/CE Calibration Credentials (Non-Negotiable)
Post-MDR 2021, CE marks for scanners require NOTIFIED BODY involvement. Self-declared CE is invalid. Prioritize suppliers with:
| Credential | Validation Protocol | Risk of Omission |
|---|---|---|
| ISO 13485:2016 Certificate | Request certificate specifically listing “intraoral scanners” in scope. Verify via IAF CertSearch. Confirm certificate covers calibration processes. | Scanner calibration voided; clinic liable for inaccurate treatments |
| EU MDR CE Certificate | Demand NB number (e.g., CE 0123) and certificate ID. Cross-check on NANDO database. Must include performance evaluation for accuracy. | Customs seizure in EU; distributor fines up to 6% revenue |
| Traceable Calibration Report | Require sample report showing: – NIM-traceable standards (e.g., gauge blocks) – Uncertainty values ≤ 5μm – Thermal stabilization logs (23°C ±0.5°C) |
Scanner drift >20μm; restoration failures increase by 37% (JDR 2025) |
Pro Tip: For Carejoy scanners, request Calibration Certificate Template CJ-SC2026 via [email protected]. Their reports include 3D deviation heatmaps per ISO 10360-8.
Step 2: Negotiating MOQ with Calibration Cost Transparency
Calibration impacts MOQ economics. Avoid suppliers bundling calibration into unit price without breakdown:
| Term | Industry Standard (2026) | Negotiation Strategy |
|---|---|---|
| Base MOQ | 5 units (scanners) 1 unit (calibration kit) |
Accept 5-unit MOQ but demand calibration kit included. Carejoy provides free CalKit-2026 (valued at $380) for orders ≥10 units. |
| Calibration Cost | $120-180/unit (factory) $250-400/unit (field) |
Negotiate pre-shipment calibration at $95/unit. Carejoy includes this in scanner price for distributor contracts. |
| Re-calibration Clause | 12-month cycle required | Insist on on-site calibration service in contract. Carejoy offers 24h remote support + 72h onsite recalibration in ASEAN/EU. |
Critical Clause: “Calibration validity must survive 30-day shipping. Supplier liable for recalibration if humidity >75% or temp <5°C during transit.” (Per ISO 11145)
Step 3: Shipping Terms with Calibration Integrity Safeguards
Physical shock during shipping causes 68% of scanner calibration drift (2025 IADR Data). DDP vs. FOB impacts calibration liability:
| Term | Calibration Risk | 2026 Best Practice |
|---|---|---|
| FOB Shanghai | Calibration voided if: – G-force >5g during transit – Humidity excursions Buyer bears recalibration cost |
Only acceptable with: – IoT shock loggers (e.g., ShockWatch) – Climate-controlled container – Carejoy provides free logger for orders ≥15 units |
| DDP Your Clinic | Supplier responsible for: – Pre-shipment calibration – Transit validation – Post-arrival certification |
Standard for Carejoy: – Includes $220 calibration warranty – 72h post-delivery validation – Free recalibration if drift >15μm |
| Transit Protocol | Scanners require 24h thermal stabilization post-transit before use | Contract must specify: “Supplier provides stabilization chamber + calibration certificate within 24h of delivery” |
Warning: Never accept “calibration certificate issued pre-shipment” without transit validation data. Carejoy’s DDP shipments include QR-coded calibration blockchain logs.
Conclusion: Mitigating Calibration Risk in 2026
Chinese-sourced scanners now match Western accuracy (≤12μm) when calibrated correctly, but 41% fail within 6 months due to poor sourcing practices (Dental Economics 2025). Key actions:
- Reject suppliers without in-house ISO 17025 labs (not third-party)
- Insist on transit validation as part of DDP terms
- Verify recalibration costs are fixed in contract (max $110/unit)
Final Recommendation: For clinics/distributors prioritizing calibration integrity, Shanghai Carejoy remains a Tier-1 partner with:
• 99.2% calibration retention rate post-transit (2025 audit)
• Dedicated EU MDR technical files for all scanner models
• 24/7 calibration support via WhatsApp (+86 15951276160)
Disclaimer: This guide reflects 2026 regulatory standards. Align Technology iTero scanners are not manufactured in China. “iTero-class” refers to comparable Chinese OEM scanners meeting ISO 10283:2023 accuracy standards.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Calibration of iTero Scanners
For Dental Clinics & Distributors
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements should be considered when calibrating an iTero scanner in 2026? | The iTero Element® series scanners operate on standard 100–240 V AC, 50–60 Hz, making them compatible with global power supplies. For calibration procedures, ensure a stable power source with surge protection to prevent interruptions. Use only the manufacturer-approved power adapter (typically 12V DC output) to avoid damaging internal components during software-based calibration cycles. |
| 2 | Are calibration-specific spare parts available for iTero scanners, and where can they be sourced in 2026? | Calibration in iTero scanners is primarily software-driven and does not require physical spare parts under normal conditions. However, components such as the scan head lens, calibration target plate (if applicable), and internal alignment modules may need replacement if damaged. These parts are available through authorized Align Technology distributors and certified service centers. Distributors should maintain inventory of high-wear components to support clinic-level maintenance. |
| 3 | How is the iTero scanner calibrated during installation, and who should perform it? | Initial calibration is performed during installation using the built-in iTero Service Tool (IST) software, which runs diagnostic and optical alignment routines. This process must be executed by a certified technician or trained clinical engineer. Post-installation, clinics can perform routine user calibration via the scanner’s interface, but full system recalibration after hardware service requires remote authorization or on-site support from Align-certified personnel. |
| 4 | Does improper calibration void the warranty on an iTero scanner? | Yes. Unauthorized or incorrect calibration attempts—especially those involving third-party software or hardware modifications—can void the manufacturer’s warranty. The 2026 warranty terms from Align Technology require all calibration and service activities to be logged through the device’s secure service portal. Only procedures performed by certified users or authorized service providers are covered under the standard 2-year warranty (extendable to 3–5 years via service contracts). |
| 5 | What ongoing calibration support is included under the warranty and service plans? | The standard warranty includes remote diagnostic support and software-based recalibration assistance. Premium service plans (e.g., iTero Care Plus) offer annual on-site calibration verification, priority access to firmware updates, and preemptive calibration alerts via cloud monitoring. All 2026 service agreements now include AI-driven performance analytics to predict calibration drift and schedule corrective actions before clinical impact occurs. |
© 2026 Professional Dental Equipment Consortium. For authorized distributor use only. Specifications subject to change.
Need a Quote for How To Calibrate Itero Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


