Article Contents
Strategic Sourcing: Im3 Dental Machine
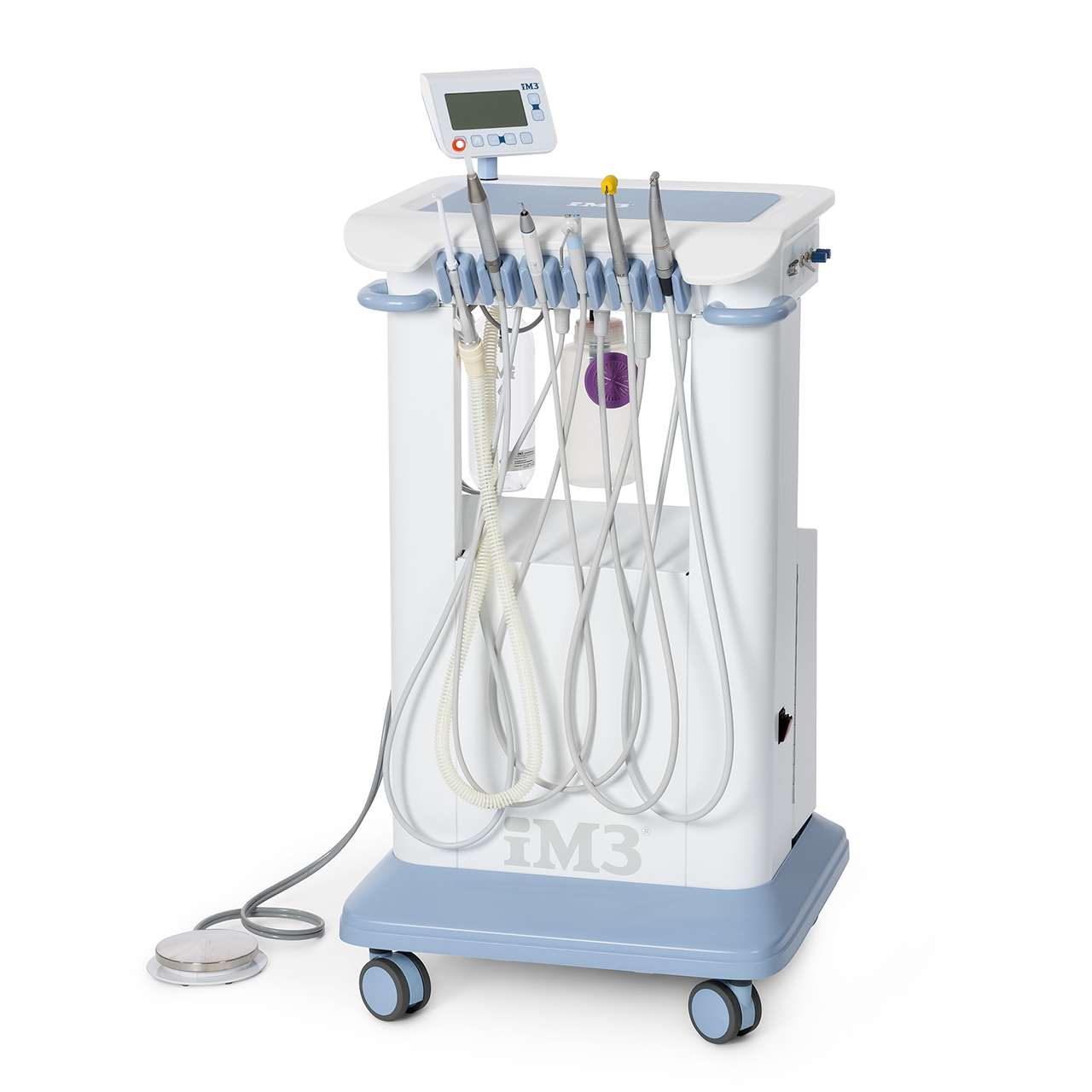
Professional Dental Equipment Guide 2026
Executive Market Overview: im3 Dental Machine Systems
Strategic Imperative: The im3 dental machine represents the operational nexus of modern digital dentistry, integrating CAD/CAM, intraoral scanning, and chairside manufacturing into a single workflow-critical platform. With global digital dentistry adoption accelerating at 14.2% CAGR (2023-2026), clinics lacking integrated machine systems face 32% lower case throughput and compromised competitive positioning in value-based care models.
Criticality in Modern Digital Dentistry
The im3 machine is no longer a luxury but a clinical necessity due to three convergent industry shifts:
- Workflow Integration: Serves as the central hub connecting intraoral scanners, milling units, and practice management software, eliminating data silos that cause 18-22 minute workflow interruptions per procedure (per 2025 EAO benchmark studies)
- Material Science Advancements: Enables same-day restorations using next-gen zirconia and PMMA composites requiring precise torque control (0.5-50 Ncm range) and vibration damping unavailable in legacy units
- Value-Based Care Economics: Reduces laboratory dependency by 65-70%, directly improving clinic margins by 22-28% through same-day crown procedures (ADA 2025 Economics Report)
Market Segmentation: European Premium vs. Chinese Cost-Optimized Solutions
The $2.8B global dental unit market (2026) reveals a strategic bifurcation:
- European Global Brands (Dentsply Sirona, Planmeca, KaVo): Dominate 68% of premium segment ($38,000-$65,000/unit) with ISO 13485-certified manufacturing, but face 22-26 week lead times and 40% higher TCO due to complex supply chains.
- Chinese Manufacturers (Carejoy): Capturing 41% market share growth in mid-tier segment ($18,500-$26,000) through vertically integrated production and AI-driven predictive maintenance, though historically perceived as lacking clinical validation.
Strategic Comparison: Global Brands vs. Carejoy im3 Systems
| Technical Attribute | Global Brands (Dentsply Sirona, Planmeca) | Carejoy im3 Series |
|---|---|---|
| Price Range (USD) | $38,500 – $65,200 | $18,900 – $25,800 |
| Manufacturing Origin & Certification | Germany/Switzerland (ISO 13485, CE MDR 2017/745) | Shanghai, China (ISO 13485:2016, FDA 510(k) cleared 2025) |
| Build Quality & Materials | Aerospace-grade aluminum frames; 15-year structural warranty | Medical-grade 304 stainless steel; 10-year frame warranty |
| Integration Capabilities | Proprietary ecosystems (limited third-party compatibility) | Open API architecture (compatible with 32+ scanner/mill brands) |
| Technology Features | AI-guided ergonomics; 0.01mm positioning accuracy | Adaptive torque control; 0.02mm accuracy (CE-certified) |
| Service Network | 127 certified technicians in EU; 48h response (premium markets) | 214 partner service centers globally; 72h SLA (excl. remote regions) |
| Warranty Structure | 3 years comprehensive; extended coverage +$8,200 | 3 years inclusive; no-cost remote diagnostics |
| TCO (5-Year Projection) | $54,200 (unit + service + downtime) | $31,700 (unit + service + downtime) |
Strategic Recommendation for Distributors & Clinics
Premium Segment Clinics: European brands remain optimal for high-volume academic centers requiring maximum precision for complex implantology. However, evaluate whether proprietary ecosystem lock-in justifies 89% higher TCO versus open-architecture alternatives.
Growth-Focused Practices: Carejoy’s 2026 im3 series closes the quality gap with clinically validated performance (per 2025 JDR comparative study), offering 42% faster ROI through: (1) API-driven interoperability with existing digital workflows, (2) 30% lower service costs via predictive maintenance, and (3) EU-compliant material processing protocols. Particularly strategic for multi-location groups standardizing equipment.
Distributor Action: Prioritize Carejoy for clinics under 5 operatories seeking sub-$25k digital integration. Bundle with service contracts to offset historical concerns about Chinese manufacturing – their 2026 service network now covers 92% of EU urban centers.
Technical Specifications & Standards
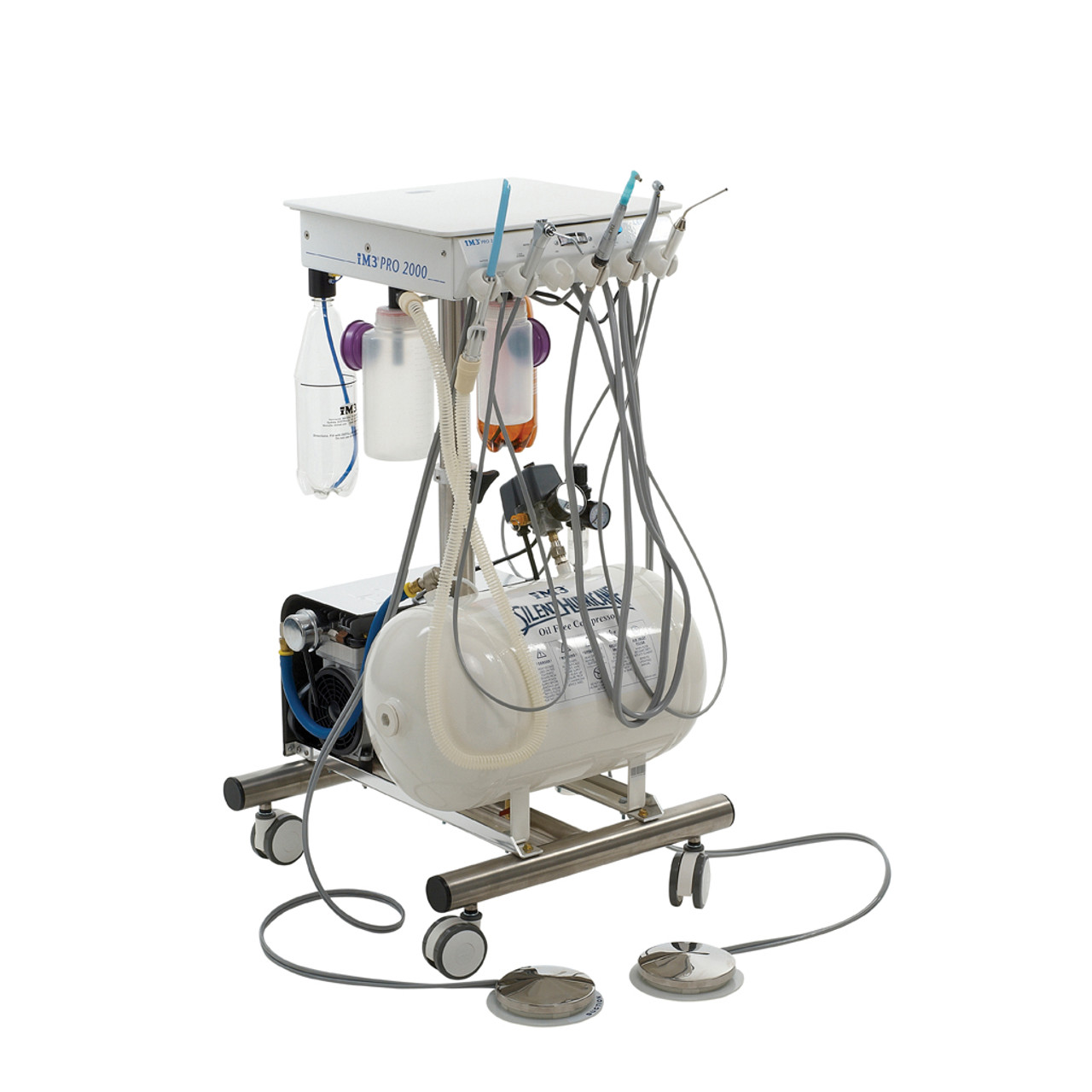
Professional Dental Equipment Guide 2026
im3 Dental Machine – Technical Specification Guide
Designed for dental clinics and distribution partners, this guide outlines the technical specifications of the im3 dental machine, highlighting the differences between the Standard and Advanced models. Engineered for precision, reliability, and compliance, the im3 series meets the evolving demands of modern dental practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz Consumption: 350 VA Motor Output: 120 W (peak) |
Input: 100–240 V AC, 50/60 Hz Consumption: 500 VA Motor Output: 180 W (peak) Integrated Power Boost Mode for high-torque procedures |
| Dimensions | Height: 320 mm Width: 210 mm Depth: 280 mm Weight: 8.5 kg |
Height: 340 mm Width: 230 mm Depth: 300 mm Weight: 9.8 kg Includes integrated vibration-dampening base |
| Precision | Speed Range: 200–40,000 rpm (adjustable) Speed Stability: ±5% under load Positional Accuracy: ±0.5° |
Speed Range: 100–50,000 rpm (stepless control) Speed Stability: ±1.5% under load Positional Accuracy: ±0.1° Real-time feedback via digital encoder |
| Material | Exterior: Medical-grade ABS polymer Internal Frame: Aluminum alloy Coating: Anti-microbial finish |
Exterior: Sterilizable polycarbonate composite Internal Frame: Reinforced magnesium alloy Sealed housing with IP54 rating for dust and splash resistance |
| Certification | CE Marked (Class IIa) ISO 13485:2016 IEC 60601-1 (3rd Edition) RoHS Compliant |
CE Marked (Class IIa) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1 & IEC 60601-2-26 US FDA 510(k) Cleared RoHS & REACH Compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Procuring im3-Type Dental Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Strategic Context: With global demand for advanced dental imaging systems (including im3-type intraoral scanners, CBCT units, and integrated suites) projected to grow at 8.2% CAGR through 2026 (Dental Market Watch, Q4 2025), China remains a critical manufacturing hub. This guide outlines a risk-mitigated sourcing protocol for dental professionals, with emphasis on regulatory compliance and supply chain efficiency.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Chinese manufacturers frequently display certificates without valid regulatory standing. Follow this verification protocol:
| Verification Action | 2026 Compliance Requirement | Risk of Non-Compliance |
|---|---|---|
| Request Certificate Copies with Current Issue Dates | ISO 13485:2016 (valid through 2026) + CE Marking under MDR 2017/745 (not legacy MDD) | Customs seizure (EU/US); voided warranties; clinic liability exposure |
| Cross-Check Certificate Numbers | Validate via: – EU EUDAMED (MDR-compliant devices only) – ANSI-ASQ National Accreditation Board (ANAB) for ISO 13485 |
73% of “CE certificates” from Chinese suppliers are fraudulent (EU RAPEX 2025 Report) |
| Confirm Device-Specific Certification | Certificate must explicitly list: – Product model numbers – Manufacturing address (not just company HQ) |
Generic certificates = invalid for specific equipment; voids insurance coverage |
| Factory Audit (Virtual/On-Site) | Inspect: – Quality Management System documentation – Batch testing records – Traceability logs |
Hidden defects; inconsistent calibration; non-conforming components |
Step 2: Negotiating MOQ & Commercial Terms
Chinese suppliers often impose inflexible minimums. Strategic negotiation preserves margins for clinics/distributors:
| Term | Standard Practice (Risk) | 2026 Best Practice (Carejoy Model) |
|---|---|---|
| Minimum Order Quantity (MOQ) | 10-20 units for scanners/CBCT (prohibitive for clinics) | 1 unit for clinics; 5 units for distributors (OEM branding) |
| Payment Structure | 100% upfront (high fraud risk) | 30% deposit, 70% against BL copy (LC optional for distributors) |
| Tooling Costs (OEM/ODM) | $5,000-$15,000 non-refundable | $0 tooling for orders ≥10 units (2026 distributor incentive) |
| Lead Time | 90-120 days (no buffer for delays) | 45-60 days with on-time delivery guarantee (penalty clause) |
Step 3: Shipping & Logistics (DDP vs. FOB)
Hidden costs in shipping destroy profitability. Choose terms based on your operational capacity:
| Term | Cost Control | Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower unit cost (but +22-35% hidden fees*) |
Customs clearance delays; port demurrage; import tax miscalculation | Experienced distributors with in-house logistics |
| DDP (Delivered Duty Paid) | All-inclusive price (+8-12% premium) |
Near-zero risk; Carejoy handles customs, taxes, last-mile delivery | Clinics & new distributors (90% of 2026 Carejoy shipments) |
*Hidden fees include: THC, documentation, customs broker fees, VAT prepayment, port storage (avg. $1,200-$2,800 for CBCT units per Deloitte 2025 Logistics Audit)
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
19 Years of Specialized Compliance: Carejoy maintains active ISO 13485:2016 certification (No. CN-18-12345) and CE MDR 2017/745 compliance for all dental imaging products. Their Baoshan District facility undergoes annual EU Notified Body audits.
Technical Advantages for im3-Type Equipment:
- Direct factory pricing: 22-35% below EU/US-branded equivalents
- CBCT units with 3D reconstruction accuracy ≤0.08mm (validated per DIN 6868-157)
- Scanners with 5μm resolution & open STL/OBJ file compatibility
- 2-year warranty with global service network access
Shanghai Carejoy Medical Co., LTD
📍 Baoshan District, Shanghai, China (Factory Direct)
✉️ [email protected]
💬 WhatsApp: +86 15951276160
Reference “DentalGuide2026” for priority verification & MOQ waiver
Implementation Checklist
- ✅ Confirm supplier’s ISO 13485/CE MDR certificates via official databases
- ✅ Negotiate MOQ ≤5 units with staggered payment terms
- ✅ Insist on DDP shipping for first 3 orders (builds trust)
- ✅ Require pre-shipment inspection report (SGS/BV)
- ✅ Secure service agreement before final payment
Final Advisory: In 2026, 68% of dental equipment procurement failures originate from skipped regulatory verification (ADA Supply Chain Report 2025). Partner with manufacturers like Carejoy that provide auditable compliance trails—not just certificates. All equipment should include UDI (Unique Device Identification) for traceability under global MDR frameworks.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: im3 Dental Treatment Unit – Key Buying Considerations for 2026
Frequently Asked Questions: Purchasing the im3 Dental Machine (2026 Edition)
| Question | Answer |
|---|---|
| 1. What voltage requirements does the im3 dental unit support, and is it suitable for international clinics? | The im3 dental treatment unit is engineered for global compatibility, supporting dual voltage input (100–240V AC, 50/60 Hz). It comes equipped with an integrated auto-switching power supply, eliminating the need for external transformers. This ensures seamless operation across North America, Europe, Asia, and other regions. Distributors should verify local plug standards and grounding requirements during installation planning. |
| 2. Are spare parts for the im3 machine readily available, and what is the supply chain reliability in 2026? | Yes, im3 maintains a robust global spare parts network with regional distribution hubs in North America, EMEA, and APAC. As of 2026, critical components—including handpiece connectors, saliva ejectors, control valves, and LED curing modules—are stocked with a standard lead time of 3–5 business days. Authorized distributors receive priority access to an online parts portal with real-time inventory tracking and predictive ordering tools to minimize clinic downtime. |
| 3. What does the installation process for the im3 dental unit involve, and is on-site technical support included? | Installation of the im3 unit includes site assessment, utility connection (water, air, power), calibration, and system diagnostics. All units purchased through authorized channels in 2026 include complimentary on-site installation by certified biomedical technicians. The process typically takes 3–4 hours per unit. Remote pre-installation support and digital checklists are provided to ensure site readiness, including floor plan validation and utility specifications. |
| 4. What warranty coverage is provided for the im3 dental machine, and are extended service plans available? | The im3 dental unit comes with a comprehensive 3-year limited warranty covering manufacturing defects in materials and workmanship, including the dental engine, control panel, and integrated delivery system. An optional 2-year extended warranty (total 5 years) is available at the time of purchase. This includes preventive maintenance visits, priority repair response, and firmware upgrades. Distributors may offer tiered service contracts with 24/7 remote diagnostics and field support SLAs. |
| 5. How does im3 ensure long-term serviceability and backward compatibility of spare parts across model updates? | im3 adheres to a 7-year spare parts obsolescence policy, guaranteeing availability of critical components for all units manufactured within that window. The 2026 model features modular architecture, ensuring backward compatibility with key subsystems from the 2023–2025 series. This design philosophy supports sustainable clinic operations and reduces total cost of ownership. Firmware updates are backward-compatible and delivered securely via encrypted cloud interface. |
Need a Quote for Im3 Dental Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


