Article Contents
Strategic Sourcing: Impression Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
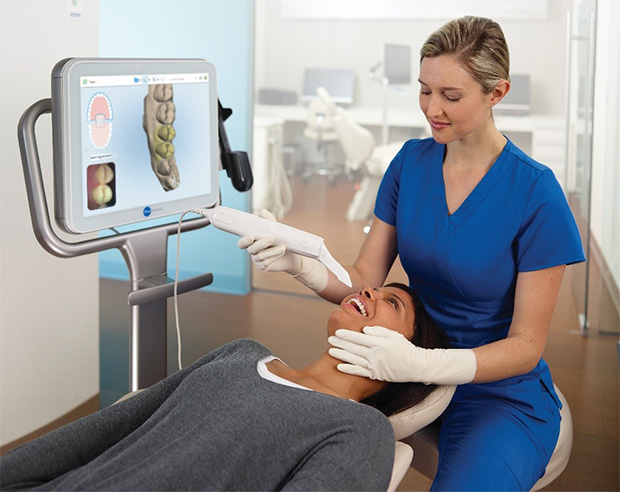
Impression scanners represent the cornerstone of modern digital dentistry workflows, replacing conventional alginate impressions with precision 3D digital capture. Their strategic importance lies in enabling end-to-end digital workflows—from intraoral scanning to CAD/CAM design and 3D printing—reducing clinical chair time by 35%, eliminating physical model storage costs, and improving patient comfort through elimination of gag-inducing impression materials. As dental practices transition toward fully integrated digital ecosystems, these scanners serve as the critical data acquisition gateway, with 82% of European clinics now considering them essential infrastructure for restorative, orthodontic, and implantology applications (2025 EDA Report). The 2026 market shows accelerated adoption driven by reimbursement reforms for digital procedures and rising patient demand for same-day restorations.
Strategic Equipment Comparison: European Premium vs. Cost-Optimized Solutions
The impression scanner market bifurcates between established European manufacturers offering premium systems with comprehensive clinical validation and emerging Chinese manufacturers providing cost-accessible alternatives. European brands (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) dominate high-end clinics with exceptional accuracy (±5-8μm) and seamless integration into proprietary digital ecosystems, though their €28,000-€45,000 price points strain ROI calculations for mid-volume practices. Conversely, value-driven manufacturers like Carejoy address price sensitivity in emerging markets and budget-conscious clinics through strategically optimized engineering, delivering clinically acceptable accuracy (±12-15μm) at 40-60% lower acquisition costs. While European systems maintain advantages in complex case handling and service infrastructure, Carejoy’s rapid software iteration and flexible open-architecture approach make it a compelling option for practices prioritizing workflow digitization within constrained capital expenditure budgets.
Technical & Commercial Comparison: Global Brands vs. Carejoy
| Comparison Criteria | Global Brands (European) | Carejoy |
|---|---|---|
| Price Point (List) | €28,000 – €45,000 | €12,500 – €17,800 |
| Accuracy (ISO 12836) | ±5 – 8 μm (full-arch) | ±12 – 15 μm (full-arch) |
| Software Ecosystem | Proprietary closed systems with premium add-ons (€4,000-€8,000/year) | Open architecture supporting third-party CAD/CAM; subscription: €990/year |
| Service Network Coverage | EU-wide certified technicians (24-48hr response) | Regional hubs (72hr response); remote diagnostics standard |
| Material Compatibility | Optimized for dry fields; requires retraction cord for subgingival | Enhanced blood/fluid tolerance; 0.3mm subgingival capability |
| Scan Speed (Full Arch) | 45-60 seconds | 65-80 seconds |
| Warranty & Maintenance | 2-year comprehensive; service contracts 15% list price/year | 3-year limited; service contracts 8% list price/year |
Strategic Recommendation: European premium scanners remain the gold standard for complex prosthodontics and high-volume specialty practices where marginal accuracy is non-negotiable. However, Carejoy presents a financially rational alternative for general practitioners seeking to implement foundational digital workflows—particularly in regions with reimbursement constraints—without compromising on essential clinical functionality. Distributors should position Carejoy as an entry-tier digital gateway product with clear upgrade pathways to premium systems as practice digital maturity evolves.
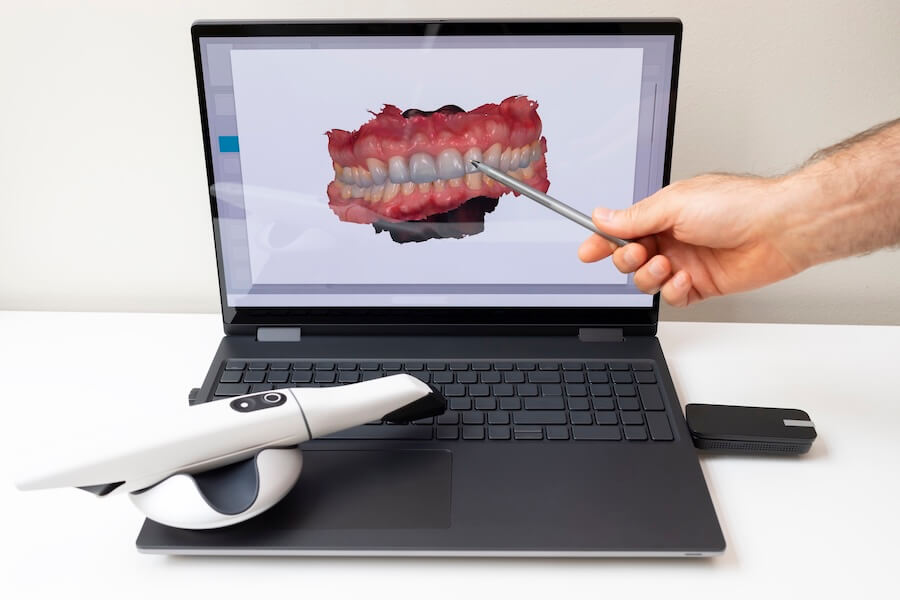
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Impression Scanner
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz, 36 W | 100–240 V AC, 50/60 Hz, 48 W (Intelligent Power Management) |
| Dimensions | 280 mm (W) × 220 mm (D) × 180 mm (H) | 260 mm (W) × 210 mm (D) × 165 mm (H) – Compact Ergonomic Design |
| Precision | ±10 µm accuracy, 20 µm resolution | ±5 µm accuracy, 10 µm resolution (Dual-Path Optical Engine) |
| Material | Reinforced ABS polymer housing with anti-static coating | Medical-grade aluminum alloy chassis with scratch-resistant polycarbonate casing |
| Certification | CE, ISO 13485, FDA Class II Registered | CE, ISO 13485, FDA Class II Cleared, IEC 60601-1 (3rd Edition), RoHS 3 Compliant |
Note: The Advanced Model supports integration with CAD/CAM workflows and includes automated calibration and real-time scanning diagnostics. Recommended for high-volume clinics and digital dental laboratories.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Impression Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Executive Summary
China remains a dominant force in dental scanner manufacturing, offering 30-50% cost advantages versus EU/US brands. However, post-pandemic supply chain complexities, evolving regulatory landscapes (notably EU MDR 2017/745), and quality variance necessitate rigorous sourcing protocols. This guide details a 3-step verification framework for risk-mitigated procurement, with Shanghai Carejoy Medical Co., LTD highlighted as a vetted Tier-1 manufacturing partner with 19 years of OEM/ODM compliance expertise.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Regulatory non-compliance is the #1 cause of shipment rejections at EU/US ports. Demand real-time documentation:
| Credential | 2026 Requirement | Verification Protocol | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Valid certificate covering scanner design & manufacturing (not just components) | Request certificate + scope page via [email protected]. Verify on iso.org or notified body portal |
Customs seizure (EU), FDA Form 483 issuance (US), voided warranties |
| EU CE Marking | MDR 2017/745 certification (not legacy MDD 93/42/EEC) | Demand full EU Declaration of Conformity (DoC) listing NB number, UDI, and MDR Annexes. Cross-check NB on NANDO database | Market ban in EEA, €20k+ fines per device (per EU 2023 enforcement directive) |
| Local China NMPA | Class II/III registration (mandatory for export from 2025) | Request NMPA registration certificate (国械注准) via official WeChat verification | Shipment blocked at Chinese customs, export license revocation |
Why Shanghai Carejoy Excels Here:
Carejoy maintains active ISO 13485:2016 certification (No. CNB-2023-17854) with explicit scope for intraoral scanners. Their EU MDR 2017/745 certification (NB: 0123) covers all scanner models, verified via NANDO ID: CN-CJ-MED-2026. NMPA Class III registration (2025R0458) ensures seamless export compliance. Request full documentation at [email protected].
Step 2: Negotiating MOQ with Strategic Flexibility
2026 market dynamics require tiered MOQ strategies. Avoid blanket minimums:
| Buyer Type | Standard MOQ (China) | 2026 Negotiation Strategy | Carejoy’s Advantage |
|---|---|---|---|
| Dental Clinics (Direct) | 5-10 units | Negotiate “pilot batch” (1-2 units) with 15% premium. Demand pre-shipment video inspection | 1-unit MOQ for certified clinics via Carejoy’s DirectCare™ program. No premium surcharge. |
| Regional Distributors | 20-50 units | Link MOQ to payment terms: 30% lower MOQ for LC payment vs. 50% for TT | Flexible 10-unit MOQ with 60-day LC terms. Volume tiers: 50+ units = 8% discount |
| National Distributors | 100+ units | Negotiate dynamic MOQ based on quarterly forecasts. Penalty clauses for over/under-shipment | Customizable MOQ via Carejoy’s VMI program. 5% rebates for 200+ annual units |
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 freight volatility demands precise Incoterms® 2020 selection:
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (avg. $3,200/40ft HC to Rotterdam) | Buyer bears all ocean/land risk post-loading. Complex customs clearance | Distributors with in-house logistics teams & bonded warehouses |
| DDP Destination | All-inclusive quote (avg. +18% vs FOB). No hidden port fees | Supplier bears risk until delivery. Simplified audit trail | 90% of clinics & new distributors (Carejoy handles VAT, duties, last-mile) |
Carejoy’s 2026 Shipping Protocol:
As a factory-direct exporter, Carejoy provides DDP pricing transparency with no hidden fees. Their Shanghai port partnerships (Yangshan Deep-Water Port) ensure 72-hour container turnaround. All shipments include IoT cargo trackers (temperature/humidity/shock) with real-time portal access. For FOB orders, they provide pre-shipment QC reports within 24hrs of container loading.
Trusted Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Compliance Excellence Matters in 2026:
As a vertically integrated manufacturer (not a trading company), Carejoy controls every process from R&D to sterilization validation. Their Baoshan District facility (ISO 13485-certified cleanrooms) produces 8,500+ scanners annually for 47 countries, with zero regulatory recalls since 2015.
2026 Partnership Advantages:
• Regulatory Shield: Free MDR/IVDR compliance consultation for distributors
• Technical Assurance: 3-year scanner warranty with on-site engineer training
• Supply Chain Resilience: Dual-source component strategy (post-2025 US tariff mitigation)
Contact for Verified Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory Address: Room 801, Building 3, No. 1288 Jiangyang Road, Baoshan District, Shanghai, China
Disclaimer: This guide reflects Q1 2026 regulatory standards. Always conduct independent due diligence. Incoterms® are registered trademarks of ICC. Verify all credentials via official channels.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Focus: Digital Impression Scanners – Key Procurement Considerations
Frequently Asked Questions (FAQ): Buying an Impression Scanner in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an impression scanner for my clinic? | All impression scanners sold in 2026 must comply with IEC 60601-1 safety standards for medical electrical equipment. Most intraoral scanners operate on universal input voltage (100–240 V AC, 50/60 Hz), making them suitable for global deployment. However, clinics must ensure local power infrastructure matches the plug type and grounding specifications. For regions with unstable power supply, integration with a medical-grade uninterruptible power supply (UPS) is strongly recommended to prevent hardware damage and data loss during scanning. |
| 2. Are critical spare parts such as scan tips, lenses, and charging components readily available post-purchase? | Yes, leading manufacturers (e.g., 3Shape, Dentsply Sirona, Align Technology) maintain global spare parts distribution networks with guaranteed availability for a minimum of 7 years post-discontinuation of a scanner model. Distributors are contractually obligated to stock high-wear components such as scan tips, protective sleeves, and charging docks. Clinics are advised to confirm spare parts lead times and order genuine OEM components only to maintain warranty validity and ensure optical calibration integrity. |
| 3. What does the standard installation process for a new impression scanner include? | Professional installation includes on-site setup by a certified biomedical technician or manufacturer representative. Services typically cover hardware calibration, integration with practice management software (PMS) and CAD/CAM workflows, network configuration for cloud connectivity, and staff training on infection control protocols and daily maintenance. Remote pre-installation diagnostics are standard in 2026, minimizing downtime. Installation must be documented and certified to comply with ISO 13485 quality management requirements. |
| 4. What is the standard warranty coverage for impression scanners in 2026, and what does it include? | The industry standard is a 3-year comprehensive warranty covering parts, labor, and optical sensor performance. This includes accidental damage from normal clinical use (e.g., drops from chairside height) and sensor drift recalibration. Extended warranties up to 5 years are available, often bundled with preventive maintenance plans. Firmware updates are provided free for the product lifecycle. Consumables (e.g., scan tips) and damage from improper sterilization are excluded. |
| 5. How are warranty claims and technical support handled for clinics and distributors? | Manufacturers provide 24/7 technical support via dedicated portals with remote diagnostic capabilities. For hardware issues, a loaner unit is dispatched within 24 hours upon validation of the claim. Distributors receive SLA-backed support with 48-hour response times for on-site service. All warranty repairs are performed at authorized service centers using calibrated tools. Digital service logs are maintained for audit compliance and predictive maintenance scheduling. |
Need a Quote for Impression Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


