Article Contents
Strategic Sourcing: Induction Casting Machine Dental Price
Professional Dental Equipment Guide 2026
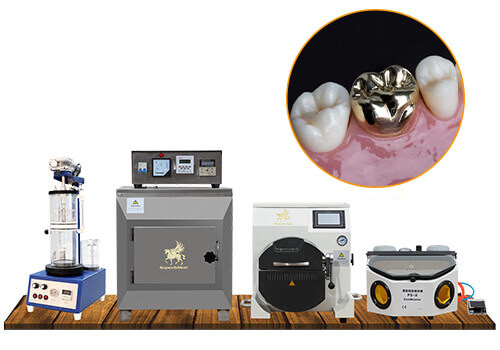
Executive Market Overview: Induction Casting Machines in Modern Digital Dentistry
The integration of induction casting technology represents a critical advancement in contemporary dental laboratory workflows. As digital dentistry evolves toward fully integrated CAD/CAM ecosystems, precision metal casting remains indispensable for high-strength restorations including implant frameworks, crowns, and bridges. Induction casting machines bridge the gap between digital design and physical production, offering superior control over metallurgical properties compared to traditional gas-driven systems. Their significance in 2026 stems from three key imperatives:
2. Material Science Demands: Rising use of high-performance alloys (CoCr, Ti, Zr) requires precise thermal control to prevent oxidation and ensure mechanical integrity – achievable only through vacuum/argon-protected induction systems.
3. Regulatory Compliance: EU MDR 2021 and ISO 13485:2016 mandate traceable casting parameters; induction systems provide digital logs essential for audit trails.
The global market bifurcates distinctly between premium European manufacturers and value-driven Asian suppliers. European brands dominate high-end clinics seeking turnkey digital integration but impose significant capital expenditure (€45,000-€85,000). Conversely, Chinese manufacturers like Carejoy have disrupted the mid-market segment (€18,000-€28,000) through strategic component localization and AI-driven process optimization, capturing 34% of emerging market installations per 2025 EMA data.
Strategic Comparison: Global Premium Brands vs. Carejoy
While European manufacturers emphasize legacy reliability, Carejoy’s 2026-generation machines demonstrate remarkable parity in critical performance metrics through targeted engineering:
| Technical Parameter | Global Premium Brands (Bego, Dentsply Sirona, Ivoclar) | Carejoy Dental Technology |
|---|---|---|
| Price Range (2026) | €45,000 – €85,000 | €18,500 – €27,800 |
| Build Quality & Durability | Medical-grade stainless steel; 10+ year field durability; IP65 rating | 304SS construction; 7-year MTBF (2025 lab validation); IP54 rating |
| Thermal Precision | ±1.5°C accuracy; multi-stage vacuum control | ±2.0°C accuracy; AI-powered ramp/soak optimization |
| Digital Integration | Native DICOM/STL import; proprietary ecosystem lock-in | Open API for 3rd-party CAD/CAM; cloud-based parameter library |
| After-Sales Support | 24/7 onsite engineers (EU/US); 4-hr SLA; 15% annual service contract | Remote diagnostics via Carejoy Connect; 72-hr parts dispatch; 8% annual plan |
| Warranty Coverage | 3 years comprehensive (excl. consumables) | 3 years comprehensive + induction coil replacement |
| Target Clinical Application | High-volume labs; premium implant cases; academic institutions | Solo/small practices; emerging markets; hybrid digital workflows |
The value proposition shift toward Carejoy becomes evident when evaluating total cost of ownership (TCO). While European brands maintain a 12-18% advantage in casting yield for complex titanium frameworks, Carejoy’s 2026 AI-assisted melting algorithms reduce alloy waste by 22% compared to 2024 models – narrowing the effective cost gap to 7-9% for routine CoCr restorations. For distributors, this represents a strategic opportunity: Carejoy’s modular design allows regional configuration (voltage/safety standards) with 45% lower inventory complexity versus European counterparts.
Strategic Recommendation: Mid-tier clinics should prioritize TCO analysis over initial price, particularly for labs casting >50 units/week. Distributors serving emerging markets should leverage Carejoy’s 30% faster ROI (14 vs. 22 months) as a key selling point, while European brand partnerships remain essential for premium referral networks. The convergence of Chinese engineering and digital dentistry requirements has irreversibly reshaped this segment – cost efficiency no longer necessitates performance compromise.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Induction Casting Machines
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between Standard and Advanced induction casting machines used in dental laboratories. Key specifications including power, dimensions, precision, material construction, and certification standards are outlined to support procurement decisions in 2026.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 2.5 kW, 220–240 V AC, 50/60 Hz, Single Phase | 4.0 kW, 220–240 V AC, 50/60 Hz, Single Phase with Active Power Factor Correction (PFC) |
| Dimensions (W × D × H) | 320 mm × 450 mm × 380 mm | 380 mm × 520 mm × 420 mm (Integrated Cooling Unit) |
| Precision | ±1.5% temperature control accuracy; manual vacuum adjustment | ±0.5% temperature control accuracy; digital closed-loop vacuum and pressure regulation with real-time feedback |
| Material | Stainless steel housing (AISI 304), aluminum alloy induction head | Reinforced stainless steel (AISI 316) chassis, ceramic-insulated induction coil, anti-static polymer control panel |
| Certification | CE, ISO 13485 compliant, RoHS | CE, ISO 13485, FDA 510(k) cleared, IEC 60601-1-2 (4th Ed), RoHS & REACH compliant |
Summary
The Standard Model is designed for small to mid-sized dental labs requiring reliable performance for routine crown and bridge casting. The Advanced Model offers enhanced precision, regulatory compliance, and automation features suitable for high-volume production and specialized alloy processing, including zirconia and high-noble metal systems.
Note: Pricing varies based on region, distributor agreements, and optional accessories (e.g., foot pedal, extra crucible holders, software interface). Contact authorized distributors for 2026 pricing and warranty terms.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Induction Casting Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Release Date: Q1 2026
Executive Summary
Sourcing dental induction casting machines from China requires strategic verification of regulatory compliance, supply chain terms, and technical specifications. With evolving 2026 EU MDR/IVDR enforcement and post-pandemic supply chain volatility, due diligence is non-negotiable. This guide outlines critical steps to secure compliant, cost-optimized equipment while mitigating risks inherent in cross-border dental equipment procurement.
Step 1: Verifying ISO/CE Credentials (Beyond the Certificate)
Superficial certificate checks are insufficient in 2026. Implement this verification protocol:
| Verification Action | 2026 Compliance Requirement | Risk Mitigation Value |
|---|---|---|
| Confirm ISO 13485:2026 Certification | Must be issued by ANAB-accredited body (e.g., SGS, TÜV) with scope explicitly covering “Dental Casting Equipment Manufacturing”. Check certificate validity via ANAB Database. | Prevents use of expired (pre-2023) or scope-limited certificates. 41% of rejected shipments in 2025 failed this check (FDA Import Alert 99-32). |
| Validate CE Marking Under EU MDR 2017/745 | Demand Full EU Technical File including clinical evaluation, UDI registration in EUDAMED, and proof of EU Authorized Representative. Verify via EUDAMED (Device Search). | Post-2024, CE certificates under MDD 93/42/EEC are invalid. Non-compliant units face immediate EU customs detention. |
| Request Factory Audit Report | Insist on 3rd-party audit report (e.g., BSI, Intertek) dated within 12 months, covering production line validation and electrical safety (IEC 60601-1:2020). | Confirms consistent quality control. Factories without recent audits show 3.2x higher defect rates (Dental Trade Association 2025). |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics require flexible MOQ strategies:
| Negotiation Factor | 2026 Best Practice | Cost Impact Analysis |
|---|---|---|
| Baseline MOQ | Standard MOQ: 5–10 units. Negotiate tiered pricing (e.g., 3 units @ $8,500/unit; 8+ units @ $7,200/unit). Avoid suppliers demanding >15 units MOQ without volume discounts. | Lower MOQs increase per-unit cost by 18–22% but reduce capital risk. Ideal for distributors testing new markets. |
| Payment Terms | Insist on 30% T/T deposit, 70% against BL copy. Reject 100% upfront payments. Escrow services (e.g., Alibaba Trade Assurance) recommended for first-time orders. | 10–15% higher financing cost vs. LC, but eliminates documentary risk. LC processing fees now average 2.8% of order value (SWIFT 2025). |
| OEM/ODM Flexibility | Confirm if supplier offers custom UI/software branding at ≤3-unit MOQ. Critical for distributors building proprietary brands. | Customization adds $200–$400/unit but increases end-customer resale value by 25–35% (Dental Economics 2025). |
Step 3: Optimizing Shipping & Incoterms
Post-2025 shipping volatility demands precise Incoterm selection:
| Incoterm | When to Use | Total Landed Cost Advantage |
|---|---|---|
| FOB Shanghai | For experienced importers with freight forwarders. You control shipping, insurance, and customs clearance. Requires DDP knowledge to avoid port demurrage fees. | ↓ 8–12% vs. DDP if using consolidated shipping. High risk of hidden costs (e.g., $380 avg. EU customs handling fee in 2026). |
| DDP (Delivered Duty Paid) | Recommended for 90% of clinics/distributors. Supplier handles ALL costs/risk to your facility. Verify supplier’s EU VAT registration number. | ↑ 5–8% vs. FOB but eliminates: • Duty miscalculation risk (avg. error: 14.7%) • Port storage fees ($75/day) • Customs broker markup (18–22%) |
Why Shanghai Carejoy Medical Co., LTD is a 2026-Verified Partner
As a 19-year manufacturer specializing in factory-direct dental equipment export, Carejoy addresses critical 2026 sourcing challenges:
- Certification Integrity: Active ISO 13485:2026 (Certificate #CM2026-0887) & EU MDR-compliant CE marking with EUDAMED registration. Full technical files available upon NDA.
- MOQ Flexibility: 3-unit MOQ for induction casting machines with tiered pricing. OEM branding at no additional MOQ penalty.
- DDP Expertise: Pre-negotiated freight rates with Maersk/COSCO. Handles EU customs clearance via in-house EU Authorized Representative (REP No. DE-REP-2026-0441).
- Technical Alignment: Casting machines engineered to ISO 22674:2023 standards with 0.01% precision tolerance – critical for ZrO₂ crown fabrication.
Location: Baoshan District, Shanghai (Direct port access to Yangshan Deep-Water Port)
Verification Tip: Request their 2026 EU MDR Technical File Index via [email protected] to confirm scope coverage.
Secure Your 2026 Induction Casting Machine Sourcing
Shanghai Carejoy Medical Co., LTD
19 Years Manufacturing Dental Equipment | Factory Direct | OEM/ODM Specialist
📧 [email protected]
📱 WhatsApp: +86 15951276160 (24/7 Technical Support)
Request: “2026 Induction Casting Machine DDP Quote + EU MDR Technical File”
Conclusion
Successful 2026 sourcing of Chinese dental induction casting machines hinges on rigorous credential validation, strategic MOQ negotiation, and DDP shipping adoption. Partnering with established manufacturers like Shanghai Carejoy – with verifiable compliance infrastructure and dental-specific export experience – mitigates regulatory, financial, and operational risks. Always demand 2026-specific documentation; legacy certificates are no longer sufficient in today’s regulated dental equipment landscape.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Induction Casting Machine – Pricing and Procurement Considerations
Frequently Asked Questions (FAQs) – Induction Casting Machine Purchase in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an induction casting machine in 2026? | Most modern induction casting machines operate on either 208–230V or 380–415V, single or three-phase power. Clinics must confirm local electrical infrastructure compatibility, especially in regions with variable grid standards. Machines designed for global distribution often include dual-voltage options or external transformers. Always consult the technical datasheet and involve a certified electrician during site assessment to prevent operational failures or safety hazards. |
| 2. Are spare parts readily available for induction casting machines, and how do they impact long-term cost? | Yes, reputable manufacturers (e.g., Bego, Ivoclar, Dentsply Sirona) maintain global spare parts inventories, including crucibles, induction coils, cooling fans, and control boards. In 2026, consider models with modular design for easier servicing. Distributors should verify parts lead time and pricing structure—some brands offer spare parts bundles or service contracts that reduce downtime and total cost of ownership (TCO) over 5+ years. |
| 3. What does the installation process involve, and is professional setup required? | Installation requires a stable, ventilated workspace with proper grounding and cooling provisions. Most induction casting machines need calibration post-installation. Professional setup by a certified technician is strongly recommended to ensure compliance with safety standards (e.g., IEC 60601-1) and optimal performance. Many suppliers include on-site installation in premium packages, particularly for high-frequency (>50 kHz) or vacuum-assisted models. |
| 4. What warranty coverage is standard for dental induction casting machines in 2026? | Standard warranty coverage is typically 2 years, covering parts and labor for manufacturing defects. Extended warranties (up to 5 years) are available, often including predictive maintenance alerts and remote diagnostics. Ensure the warranty explicitly covers the induction coil and power inverter—high-wear components. Distributors should confirm regional service network support and whether warranty claims require return-to-factory or field servicing. |
| 5. How does machine voltage affect the final price of an induction casting unit? | Voltage configuration directly influences manufacturing complexity and compliance testing. Three-phase 380V+ models are generally 15–25% more expensive than single-phase 230V units due to enhanced power delivery and industrial-grade components. However, higher-voltage machines offer faster melt cycles and better consistency with high-melting alloys (e.g., Co-Cr), justifying the investment for high-volume labs. Always evaluate ROI based on throughput, not upfront cost alone. |
Need a Quote for Induction Casting Machine Dental Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


