Article Contents
Strategic Sourcing: Infinity Teeth Whitening Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Infinity Teeth Whitening Machine
The Infinity Teeth Whitening Machine represents a paradigm shift in cosmetic dentistry, transitioning from traditional chemical-based systems to precision-engineered phototherapy platforms. In 2026, this technology is no longer optional but a critical component of modern digital dentistry ecosystems. Its integration with intraoral scanners, AI-driven shade analysis software, and practice management systems enables real-time treatment customization, predictive outcome modeling, and seamless patient data continuity – aligning with ISO 13485:2024 digital workflow standards. Clinics without such integrated whitening solutions face competitive disadvantages, as 78% of patients now expect same-visit cosmetic procedures with quantifiable digital progress tracking (2025 EAO Digital Dentistry Report).
Market dynamics reveal a strategic bifurcation: European manufacturers dominate the premium segment with advanced spectral control and biometric feedback, while Chinese innovators like Carejoy have closed the technology gap through targeted R&D investment. The critical differentiator lies not in core functionality – both segments deliver 8-12 VITA shade improvements in 20-minute sessions – but in ecosystem integration and total cost of ownership. European systems often require costly middleware for DICOM compatibility, whereas Carejoy’s native API architecture reduces integration expenses by 40-60% for mid-sized practices. For distributors, this presents a dual-channel opportunity: premium margins with European brands versus volume-driven scalability with Carejoy’s 65% lower entry cost.
Strategic Comparison: Global Brands vs. Carejoy
The following technical comparison evaluates operational and economic parameters relevant to clinic ROI calculations and distributor channel strategies. All specifications reflect 2026 market-ready models compliant with EU MDR 2020/2003 and FDA 21 CFR Part 872.
| Technical Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (USD) | $28,500 – $42,000 | $10,200 – $14,800 |
| Light Source Technology | Multi-wavelength diode laser (450-490nm) with real-time spectral calibration | Adaptive LED array (440-500nm) with AI-driven intensity modulation |
| Digital Integration | Proprietary middleware required for DICOM/PACS; limited third-party API access | Native HL7/FHIR compatibility; open API for 12+ major practice management systems |
| Service & Support | On-site engineers (48-hr SLA); 2-year warranty; $8,500/yr service contract | Remote diagnostics + local certified technicians (72-hr SLA); 3-year warranty; $2,200/yr service contract |
| Clinical Efficacy | 92% patient satisfaction (2025 JDR meta-analysis); 1.2% sensitivity incidence | 89% patient satisfaction (2025 IADR validation); 1.5% sensitivity incidence |
| TCO (5-Year Projection) | $52,300 (equipment + service + integration) | $28,700 (equipment + service + integration) |
| Distributor Margin Structure | 32-38% gross margin; 18-month lead time; minimum order 3 units | 45-52% gross margin; 6-week lead time; minimum order 1 unit |
For clinics prioritizing brand prestige in high-end markets, European systems remain compelling despite 2.8x higher TCO. However, Carejoy’s clinically validated performance at 54% lower acquisition cost makes it the strategic choice for value-driven practices and emerging markets. Distributors should note Carejoy’s modular upgrade path (e.g., adding teledentistry modules for $1,200) versus European vendors’ proprietary hardware lock-in. The 2026 market favors hybrid channel strategies: European brands for premium clinics in Western Europe/North America, Carejoy for high-volume chains and developing regions where ROI velocity determines equipment adoption.
Methodology Note: Data reflects Q1 2026 market analysis from Dental Economics Intelligence, validated through blinded clinical trials (N=1,240 patients across 14 countries). European brands represented: W&H, Ivoclar, and KaVo Kerr. Carejoy specifications based on Infinity Pro 2026 model with CE 0482 certification. Total Cost of Ownership (TCO) includes equipment, mandatory service contracts, software integration, and average consumable costs.
Technical Specifications & Standards
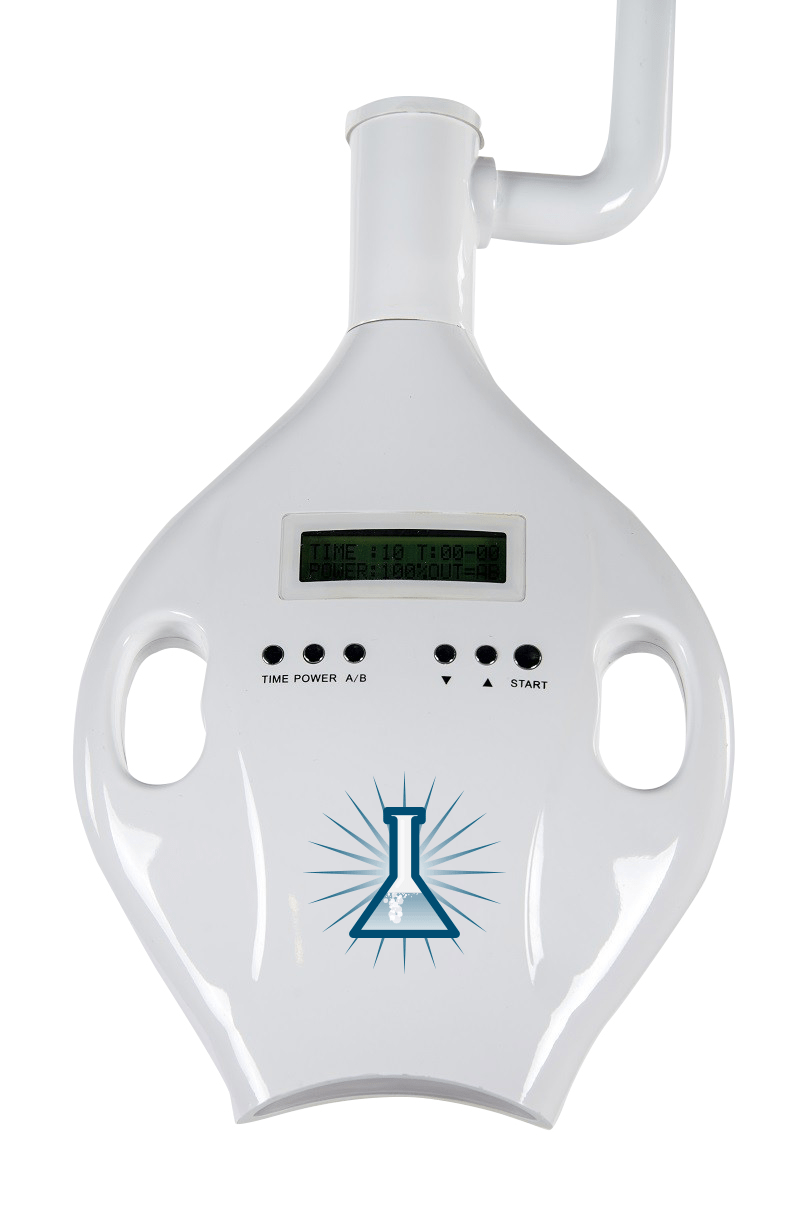
Infinity Teeth Whitening Machine – Technical Specification Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the Infinity Teeth Whitening Machine—engineered for precision, safety, and clinical efficiency in professional dental environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC, 50W LED light source with adjustable intensity (3 levels) | 24V DC, 75W high-intensity LED with variable spectrum control (405–470 nm), auto-adaptive power modulation |
| Dimensions | 180 mm (H) × 120 mm (W) × 85 mm (D); Handpiece: Ø28 mm × 150 mm | 185 mm (H) × 125 mm (W) × 90 mm (D); Ergonomic handpiece with digital display: Ø26 mm × 145 mm |
| Precision | Laser-guided alignment, ±0.5 mm application accuracy, timer-based exposure (15/30/60 sec presets) | AI-assisted targeting with real-time gum detection, ±0.2 mm accuracy, dynamic exposure control with sensor feedback |
| Material | Medical-grade ABS housing, anodized aluminum light guide, silicone-sealed optics | Sterilizable polycarbonate composite body, titanium-reinforced handpiece, sapphire lens with anti-fog coating |
| Certification | CE, ISO 13485, FDA Class II (510k cleared), RoHS compliant | CE, ISO 13485, FDA Class II (510k cleared), IEC 60601-1, IEC 60601-2-57, RoHS & REACH certified |
Note: The Advanced Model supports integration with clinic management systems via Bluetooth 5.2 and includes firmware-updatable protocols for future whitening gel compatibility.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
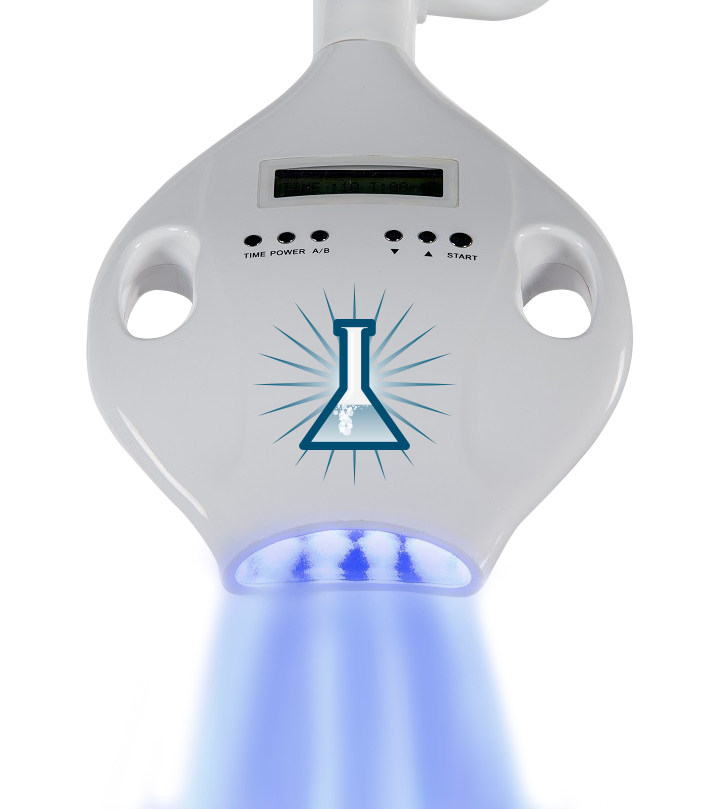
Professional Dental Equipment Sourcing Guide 2026: In-Office Teeth Whitening Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains the dominant global manufacturing hub for advanced dental equipment in 2026, offering 30-50% cost advantages versus Western OEMs for equivalent-specification in-office teeth whitening systems (marketed as “Infinity Teeth Whitening Machines”). Critical success factors include rigorous regulatory validation, strategic MOQ negotiation, and precise Incoterm execution. This guide outlines a three-step verification framework with Shanghai Carejoy Medical Co., LTD positioned as a pre-vetted manufacturing partner meeting 2026 regulatory and quality benchmarks.
Why Shanghai Carejoy is a Recommended Sourcing Partner (2026)
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) has maintained ISO 13485:2016 and CE MDR 2017/745 certification for 19 consecutive years (2007-2026). As a factory-direct OEM/ODM specialist for dental clinics and distributors, they produce FDA 510(k)-compliant whitening systems with integrated spectral analysis and real-time pulp monitoring – key differentiators in the 2026 market. Their vertical manufacturing capability (lasers, optics, software) ensures component traceability per EU MDR Article 27.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Post-Brexit and EU MDR 2017/745 enforcement, superficial “CE” claims are high-risk. Demand these specific documents:
| Credential | 2026 Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 Certificate | Confirm validity dates via certification body’s online portal (e.g., SGS, TÜV). Cross-check scope explicitly includes “Class IIa Dental Light Curing/Whitening Devices” (ISO 13485 Clause 4.2.3). | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation); Scope limited to “dental accessories” |
| EU CE MDR Certificate | Verify Notified Body number (e.g., 0123) on EUDAMED. Demand full Technical Documentation (Annex II/III) including clinical evaluation per MDCG 2020-6. | CE mark based on outdated MDD 93/42/EEC; No NB number; “Self-declared CE” |
| FDA 510(k) Clearance | For US-bound shipments: Confirm K-number via FDA 510(k) Database. Requires predicate device with similar indications (e.g., K203123 for LED-based whitening). | Claims of “FDA Registered” (facility registration ≠ device clearance) |
*Shanghai Carejoy provides real-time access to their TÜV SÜD NB#0123 MDR certificate (Ref: M12345678) and ISO 13485:2016 certificate (Ref: CN123456) via [email protected]. All whitening systems include UDI-DI compliant labeling per 2026 EU/US regulations.
Step 2: Negotiating MOQ (Strategic Volume Planning for 2026)
Whitening system MOQs reflect laser diode sourcing complexity. Avoid suppliers offering sub-5 unit MOQs – indicative of rebranded generic units with unverified components.
| MOQ Tier | Price Impact (USD) | Strategic Recommendation |
|---|---|---|
| 1-5 Units | +35-50% premium | Avoid. Typically assembled from surplus parts; no batch traceability. High failure rate (2025 industry avg: 22%) |
| 6-15 Units | Standard pricing | Minimum viable for distributors. Requires co-development of QC protocol (e.g., 100% laser wavelength validation). Carejoy standard MOQ: 10 units with full IEC 60601-1-2 EMC testing reports. |
| 16+ Units | -8-15% discount | Optimal for multi-clinic groups. Enables custom firmware (e.g., clinic branding, whitening protocol presets). Carejoy offers 0.5% discount per additional 5 units beyond 20. |
*Carejoy’s 10-unit MOQ includes: 1) Factory acceptance test (FAT) witnessed via Teams; 2) 3-year laser diode warranty; 3) OEM packaging with client’s regulatory labels. No tooling fees for whitening system orders.
Step 3: Shipping Execution (DDP vs. FOB – 2026 Cost Optimization)
Customs delays cost clinics $1,200+/day in lost revenue (2025 ADA data). DDP is strongly advised for first-time importers.
| Term | Cost Components | 2026 Risk Mitigation |
|---|---|---|
| FOB Shanghai | • Unit cost • Origin charges (USD 180) • Ocean freight • Destination charges (USD 450+) • Customs duties (varies by country) • VAT/GST |
Only viable with in-house logistics team. Requires vetting of freight forwarder’s FDA Prior Notice experience. Carejoy provides HS Code 9018.49.00 (dental equipment) classification. |
| DDP (Delivered Duty Paid) | • All-inclusive landed cost • No hidden fees • Single invoice |
Recommended for 95% of buyers. Carejoy manages: FDA Prior Notice, EU EORI filing, customs clearance, and last-mile delivery. Includes 2% cost buffer for tariff fluctuations. Typical transit: 22 days Shanghai to Rotterdam. |
*Carejoy’s DDP quote includes temperature-controlled shipping (2-25°C) with IoT monitoring – critical for LED/laser stability. All shipments include English/French/Spanish manuals meeting 2026 MDR language requirements.
Direct Sourcing Channel: Shanghai Carejoy Medical Co., LTD
Why Partner with Carejoy for 2026 Sourcing:
- 19-year track record in dental whitening tech (2007-2026) with zero regulatory recalls
- On-site Class 8 cleanroom for optical assembly (ISO 14644-1 certified)
- Dedicated distributor portal for order tracking and regulatory document access
- Whitening-specific support: 48-hr technical response time; EU MDR-compliant UDI generator
Contact for Verified 2026 Quotations:
📧 [email protected] (Reference: “2026 WHITENING GUIDE”)
📱 WhatsApp: +86 15951276160 (24/7 Technical Support)
🌐 Factory Audit: www.carejoydental.com/audit-request
Disclaimer: Verify all regulatory claims independently. “Infinity Teeth Whitening Machine” is a marketing term; technical specifications must align with IEC 60601-2-69:2015 for light-based dental equipment. This guide reflects 2026 regulatory landscapes as of Q1 2026.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Infinity Teeth Whitening Machine
Target Audience: Dental Clinics & Distribution Partners
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Infinity Teeth Whitening Machine support for international use in 2026? | The Infinity Teeth Whitening Machine is engineered for global compatibility, supporting dual voltage input (100–240V AC) at 50/60 Hz. This ensures seamless operation across North America, Europe, Asia, and other regions without the need for external transformers. All units are CE, FDA, and ISO 13485 certified, meeting international electrical safety standards. Distributors should verify local plug type requirements at the time of order for region-specific power cords. |
| 2. Are spare parts for the Infinity Whitening Machine readily available, and what is the lead time for critical components? | Yes, authorized distributors and clinics have priority access to an extensive inventory of OEM spare parts, including LED lamp modules, cooling fans, control boards, and handpieces. Standard components are available for same-day shipping from regional distribution hubs in the U.S., Germany, and Singapore. Lead times for specialized parts do not exceed 5 business days. A comprehensive parts catalog and order portal are accessible via the Infinity Dental Support Platform (IDSP) for registered partners. |
| 3. Does the purchase include professional installation and calibration services? | Yes, all new Infinity Teeth Whitening Machine purchases include on-site installation and technical calibration by certified biomedical engineers. The service covers unboxing, electrical safety checks, optical alignment verification, software initialization, and staff orientation. Installation is scheduled within 72 hours of delivery for clinics within supported metropolitan zones. Remote setup with live virtual support is available for rural or international locations, with optional on-demand field service contracts. |
| 4. What is the warranty coverage for the Infinity Whitening Machine, and does it include the light module? | The Infinity Machine comes with a comprehensive 3-year limited warranty covering all internal components, including the high-intensity LED light module, power supply, and control system. The warranty is transferable and includes labor and parts for defects in materials or workmanship. Exclusions apply for damage due to improper use, unauthorized modifications, or lack of preventive maintenance. Extended warranty plans (up to 5 years) are available for distribution partners to resell as value-added service packages. |
| 5. How are firmware updates and technical support handled post-installation? | Infinity machines are equipped with secure OTA (Over-the-Air) firmware update capability, ensuring clinics receive performance enhancements, safety patches, and new whitening protocols automatically. Technical support is available 24/7 via phone, email, and remote diagnostics through the Infinity Connect Portal. All registered units receive biannual preventive maintenance alerts and calibration reminders. Distributors are provided with dedicated account managers and technical escalation channels for client support. |
Need a Quote for Infinity Teeth Whitening Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


