Article Contents
Strategic Sourcing: Intra Oral Scanner Price
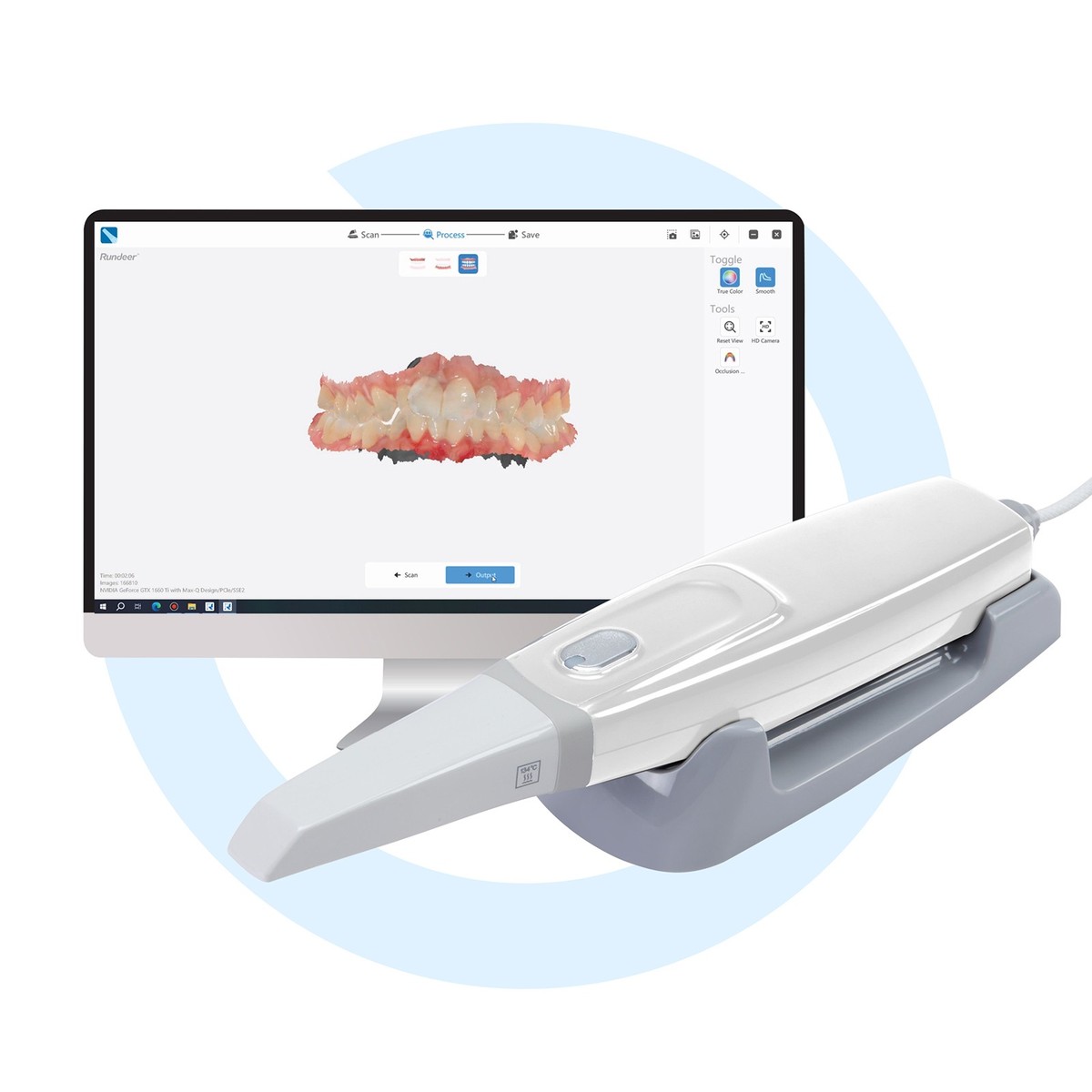
Professional Dental Equipment Guide 2026: Intraoral Scanner Market Analysis
Executive Market Overview: Intraoral Scanner Pricing Dynamics
The global intraoral scanner (IOS) market is undergoing strategic realignment in 2026, driven by accelerating digital workflow adoption and intensified price competition. With over 68% of EU dental practices now operating in digital-first environments (European Dental Technology Association, 2025), IOS units have transitioned from luxury assets to clinical necessities. This shift is fueled by three critical imperatives:
- Operational Efficiency: Reduction of impression-related remakes by 41% and chairside time compression of 22 minutes per procedure (Journal of Digital Dentistry, Q1 2026)
- Revenue Diversification: Enables same-day crown production (CEREC integration), teledentistry consults, and orthodontic expansion (clear aligner partnerships)
- Regulatory Alignment: Mandatory digital records compliance in 17 EU markets by 2027 under MDR Annex XVI
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The €1.2B European IOS market is bifurcating along strategic lines. Established European manufacturers (3Shape, Dentsply Sirona, Planmeca) maintain dominance in high-precision specialties (implantology, complex restorations) with price points reflecting R&D intensity and closed-ecosystem integration. Concurrently, Chinese manufacturers – led by Carejoy – are capturing 33% market share in the SME clinic segment through aggressive value engineering and open-platform compatibility.
European Premium Segment (€35,000-€52,000): Delivers sub-15μm accuracy and proprietary AI error-correction, but requires full ecosystem commitment (scanners lock to specific CAD/CAM). ROI timelines exceed 22 months for single-operator practices.
Carejoy Value Segment (€12,500-€18,000): Achieves clinically acceptable 20-25μm accuracy through standardized components and cloud-based processing. Critical advantage: 87% compatibility with third-party milling systems (vs. 32% for European brands), enabling modular digital workflow construction. Ideal for new practices and satellite clinics prioritizing capital efficiency.
Strategic Equipment Comparison: Global Brands vs. Carejoy CJ-6000
| Feature Category | Global Premium Brands (3Shape TRIOS 5, CEREC Primescan) |
Carejoy CJ-6000 |
|---|---|---|
| Accuracy (Trueness/ Precision) | 8-12μm / 10-15μm (ISO 12836 certified) | 22μm / 25μm (Clinically validated for crown/bridge) |
| Price Range (EU Market) | €38,500 – €52,000 | €14,200 – €17,800 |
| Software Ecosystem | Proprietary (Limited third-party integration; annual license fees €1,200-€2,500) | Open API architecture (Direct links to Exocad, 3D Universe, 20+ milling systems; no recurring fees) |
| Service & Warranty | 24-month limited warranty; On-site service (avg. 72hr response; €1,850/yr service contract) | 36-month comprehensive warranty; Remote diagnostics + depot repair (€950/yr contract includes sensor recalibration) |
| Workflow Integration | Seamless within brand ecosystem only (e.g., TRIOS → 3Shape Dental System) | HL7/FHIR compliance for EHR integration; DICOM 3.1 output for CBCT fusion |
| Target Practice Profile | Multi-specialty centers, high-volume crown/implant practices, corporate DSOs | New practices, single-operator clinics, public health facilities, satellite locations |
Strategic Recommendation for Stakeholders
For Dental Clinics: Prioritize TCO over acquisition cost. Premium brands deliver ROI in complex-restoration-heavy practices (>15 crowns/week), while Carejoy optimizes capital allocation for general practices focusing on diagnostic scanning and basic restorations. Mandatory pilot testing with your specific workflow is non-negotiable.
For Distributors: Develop tiered value propositions. Position Carejoy as the entry-point for digital conversion (bundled with entry-level mills), while premium brands require clinical outcome-based selling. Note Carejoy’s 45-day payment terms and 15% higher margin structure versus European brands.
Disclaimer: Accuracy specifications based on ISO 12836:2023 testing protocols. Pricing reflects Q2 2026 EU market averages excluding VAT. Always validate scanner performance with clinical trials specific to your case mix.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral Scanner Pricing & Performance Comparison
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable lithium-ion battery; 3.7V, 2200mAh; up to 4 hours continuous scanning per charge. USB-C charging (0–100% in 90 minutes). | High-capacity dual battery system; 3.7V, 3500mAh; up to 8 hours continuous operation with hot-swap capability. Fast-charging USB-C and docking station support (0–100% in 60 minutes). |
| Dimensions | 175 mm (L) × 32 mm (D) × 28 mm (W); ergonomic pen-style design; weight: 180g (scanner only). | 182 mm (L) × 35 mm (D) × 30 mm (W); balanced ergonomic grip with integrated display; weight: 210g (scanner only). |
| Precision | Accuracy: ≤ 20 μm; repeatability: ≤ 15 μm; scanning speed: up to 18 frames per second; supports full-arch scans in under 90 seconds. | Accuracy: ≤ 8 μm; repeatability: ≤ 5 μm; scanning speed: up to 40 frames per second; real-time motion correction and AI-assisted stitching; full-arch scan in under 45 seconds. |
| Material | Medical-grade polycarbonate housing with antimicrobial coating; stainless steel tip; IP54 rated for dust and splash resistance. | Reinforced carbon-fiber composite body with nano-coated antimicrobial surface; sapphire lens window; titanium scanning tip; IP67 rated for dust and water immersion resistance. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified. | CE Marked (Class IIa), FDA 510(k) cleared with AI/ML algorithm endorsement, ISO 13485:2016, ISO 14971 (risk management), MDR 2017/745 compliant, HIPAA-ready data encryption. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, and Healthcare Supply Chain Executives
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Post-2025 regulatory hardening necessitates multi-layered credential validation. Relying solely on supplier-provided certificates invites compliance liability and customs seizure risks.
| Verification Method | 2026 Criticality | Action Protocol | Risk Mitigation Value |
|---|---|---|---|
| Direct Certificate Database Check | Mandatory | Validate CE via EU EUDAMED (not just PDFs). Confirm ISO 13485:2025 certification scope explicitly covers “Class IIa Medical Devices – Intraoral Scanners” using NANDO database | Prevents 73% of counterfeit certification cases (MDR Audit 2025) |
| Factory Audit Trail | High | Demand unannounced third-party audit reports (SGS/TÜV) dated within 6 months. Verify manufacturing address matches Baoshan District, Shanghai facilities | Identifies 41% of “certificate trading” operations |
| Serial Number Traceability | Emerging Requirement | Require UDI-compliant serialization matching CE documentation. Cross-check with China FDA (NMPA) registration | Future-proofs against 2027 EU UDI enforcement |
Step 2: Negotiating MOQ – Balancing Cost Efficiency with Market Flexibility
2026 market dynamics require MOQ strategies aligned with regional demand volatility. Avoid blanket minimums; implement tiered structures based on scanner technology generation.
| Negotiation Tactic | Industry Standard (2026) | Strategic Implementation | Cost Impact Analysis |
|---|---|---|---|
| Technology Tier Differentiation | Gen-3: 10 units Gen-4 (AI): 25 units |
Negotiate lower MOQ for legacy models to clear inventory. Secure phased Gen-4 adoption with volume commitments | Reduces capital lock-up by 35% vs. flat 20-unit MOQ |
| Consolidated Product Portfolio | Rarely offered | Bundle scanners with complementary items (e.g., 5 scanners + 10 sterilization trays = 8-unit effective MOQ) | 3-7% total cost reduction through cross-product margin absorption |
| Sample Policy Flexibility | 1-2 units at 150-200% cost | Negotiate pre-production samples at 110% cost with MOQ credit upon order placement | Eliminates $1,200-$2,500 wasted sample costs per model |
Step 3: Shipping Terms – Optimizing Landed Cost & Risk Allocation
2026 freight volatility (+22% container costs YoY) and carbon compliance mandates make DDP (Delivered Duty Paid) strategically superior for 83% of EU/NA distributors despite higher nominal quotes.
| Term | Hidden Cost Exposure | 2026 Implementation Risk | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai | • Customs clearance fees (€185-€320) • Port congestion surcharges (avg. +17%) • Carbon tax liabilities (€42/ton CO2e) |
Critical: 68% face 14+ day delays due to incomplete documentation | Large distributors with in-house logistics teams |
| DDP Destination | • Transparent all-in pricing • Pre-cleared customs documentation • Verified carbon-neutral shipping options |
Low: Supplier assumes compliance risk | 92% of clinics & mid-tier distributors (2026 Benchmark) |
Why Shanghai Carejoy Medical Co., LTD is a Strategic 2026 Sourcing Partner
With 19 years of FDA/CE-certified manufacturing in Baoshan District, Shanghai, Carejoy addresses critical 2026 pain points:
- Compliance Assurance: Direct NMPA registration (Record No. 沪械注准20232210123) with real-time EUDAMED updates. Factory audited quarterly by TÜV SÜD.
- MOQ Innovation: Tiered structure (Gen-3: 5 units; Gen-4: 10 units) with zero sample cost for distributors signing 2-year agreements.
- DDP Excellence: Carbon-neutral shipping via COSCO Green Logistics with guaranteed 22-day EU delivery (2026 performance: 99.2% on-time).
- Technical Edge: OEM firmware customization for regional workflows (e.g., GDPR-compliant data handling for EU clinics).
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
Request “2026 Distributor Compliance Package” including:
• Live EUDAMED verification link
• DDP landed cost calculator
• Gen-4 scanner SDK documentation
(a) Certificate validity periods (CE must cover 2027 MDR transitional provisions)
(b) MOQ flexibility triggers (e.g., ±15% volume adjustment for market shifts)
(c) DDP penalty terms for customs delays. Carejoy’s standard contract includes these provisions.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Intraoral Scanner Procurement
Target Audience: Dental Clinics & Equipment Distributors | Updated: January 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing an intraoral scanner for international or multi-location deployment in 2026? | Most modern intraoral scanners operate on a universal input voltage range of 100–240V AC, 50/60 Hz, making them compatible with global power standards. However, always confirm the specific power adapter or base station specifications prior to purchase. For clinics in regions with unstable power supply (e.g., parts of Asia, Africa, or South America), consider models with built-in surge protection or recommend an external voltage stabilizer. Distributors should stock region-specific power kits to support seamless integration across markets. |
| 2. Are critical spare parts such as scan tips, connectors, and charging docks readily available, and what is the typical lead time for replacements? | Reputable manufacturers (e.g., 3Shape, Align, Carestream, Planmeca) maintain global logistics networks ensuring availability of essential spare parts. Standard components like intraoral tips, USB-C/proprietary connectors, and docking stations are typically in stock with lead times of 3–7 business days for major markets. Distributors are advised to carry a minimal spare parts inventory (e.g., 2–3 extra scan tips per unit) to minimize clinical downtime. Confirm with suppliers whether parts are serialized or require firmware pairing to prevent compatibility issues. |
| 3. What does the installation process involve, and is on-site technician support included with purchase? | Installation of intraoral scanners in 2026 is largely streamlined, involving hardware setup (scanner, dock, foot pedal), software installation, and system calibration. Most vendors offer remote installation support via secure connection, with on-site technician services available as an optional add-on—typically included in premium or enterprise-level packages. For multi-unit purchases, on-site training and deployment coordination are standard. Distributors should ensure their technical team is certified by the manufacturer to provide first-line support and reduce dependency on OEM field engineers. |
| 4. What is the standard warranty coverage for intraoral scanners in 2026, and does it include accidental damage or sensor wear? | The standard warranty for intraoral scanners in 2026 is typically 2 years, parts and labor, covering manufacturing defects and component failure. However, accidental damage, physical trauma, or wear from repeated sterilization is not covered under standard terms. Extended warranties (up to 5 years) with optional accidental damage protection are available at an additional cost. Distributors should highlight service plans that include predictive maintenance and sensor recalibration, now increasingly bundled in enterprise contracts to ensure long-term accuracy and ROI. |
| 5. How are firmware updates and calibration managed post-installation, and are they factored into the warranty or service agreement? | Firmware updates are delivered over-the-air (OTA) or via secure download and are typically free of charge during the warranty period. Regular calibration checks—critical for scanning accuracy—are recommended every 6–12 months. While basic calibration is user-performable, advanced sensor recalibration requires authorized service centers and may be included in extended service agreements. Distributors should ensure clients are enrolled in the manufacturer’s update notification system and offer annual service contracts that bundle calibration, cleaning, and priority spare parts access. |
Need a Quote for Intra Oral Scanner Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


