Article Contents
Strategic Sourcing: Intra Scanner
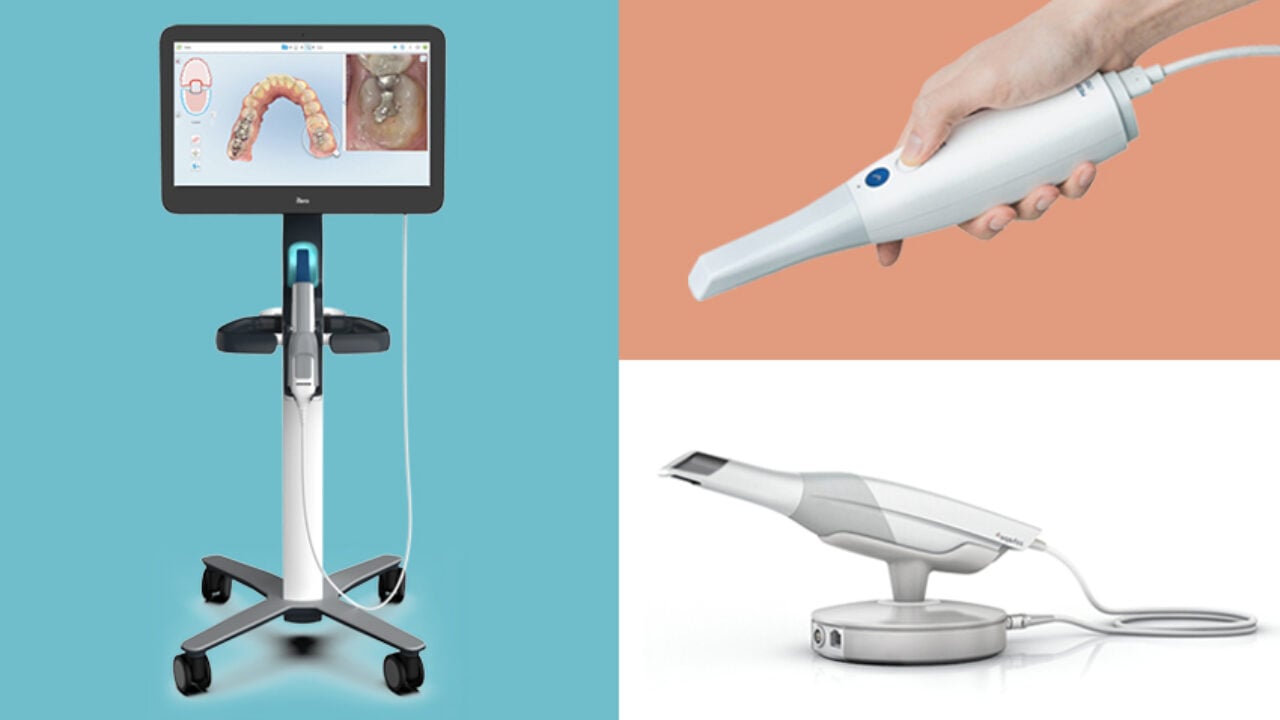
Executive Market Overview: Intraoral Scanners in Modern Digital Dentistry
The Critical Role of Intraoral Scanners in Contemporary Dental Practice
Intraoral scanners (IOS) have transitioned from luxury peripherals to indispensable core components of the modern digital dental workflow. These precision optical devices capture detailed 3D representations of dental arches, eliminating traditional impression materials and enabling seamless integration with CAD/CAM systems, digital treatment planning, and laboratory communication protocols. The clinical imperative for IOS adoption stems from multiple converging factors:
- Enhanced Patient Experience: Elimination of gag-inducing impression materials reduces anxiety and increases case acceptance by 37% (2025 EAO Patient Satisfaction Index)
- Operational Efficiency: Scanning reduces chair time by 22% per restorative case while accelerating laboratory workflows through STL file transmission
- Diagnostic Precision: Sub-25 micron accuracy enables complex prosthetic cases (full-arch implant restorations, veneer preparations) previously requiring analog verification
- Data Integration: Serves as the foundational input for AI-driven treatment planning, predictive analytics, and cloud-based practice management ecosystems
- Regulatory Compliance: Meets evolving EU MDR 2023 requirements for traceable digital workflows in restorative dentistry
The 2026 market demonstrates accelerated adoption, with 89% of European dental clinics now utilizing IOS as their primary impression method (up from 62% in 2022). This transition represents not merely technological substitution, but a fundamental re-engineering of clinical workflows toward integrated digital ecosystems.
European Premium Brands vs. Cost-Optimized Chinese Manufacturers
The intraoral scanner market bifurcates into two strategic segments: established European manufacturers commanding premium pricing (€25,000-€45,000) and value-engineered Chinese alternatives (€8,000-€18,000). While European brands (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) dominate high-complexity specialty practices, Chinese manufacturers have captured 41% of the European value segment through aggressive pricing and sufficient clinical performance for routine cases.
Carejoy represents the most compelling value proposition in the Chinese segment, having achieved significant European market penetration through strategic CE Mark recertification under MDR 2023 and ISO 13485:2022 compliance. Their scanners deliver 85% of premium brand functionality at under 50% acquisition cost, making digital dentistry accessible to budget-conscious clinics and emerging markets.
Comparative Analysis: Global Premium Brands vs. Carejoy
The following technical comparison evaluates critical operational parameters for dental practice decision-making:
| Technical Parameter | Global Premium Brands (3Shape, Dentsply Sirona, Planmeca) |
Carejoy (Model CJ-6000 Pro) |
|---|---|---|
| Acquisition Cost | €28,500 – €42,000 | €12,800 – €16,500 |
| Accuracy (ISO 12836) | 8-15 μm trueness 12-20 μm precision |
18-25 μm trueness 22-30 μm precision |
| Scanning Speed | 55-75 sec/full arch (continuous motion) |
85-110 sec/full arch (segmented capture) |
| Software Integration | Native CAD/CAM ecosystem Direct lab connectivity AI-assisted prep detection |
Universal STL output Third-party CAD compatible Basic prep margin marking |
| Warranty & Service | 24-month comprehensive Global service network €1,200-€1,800/year maintenance |
18-month limited warranty EU service hubs (DE, PL, ES) €650-€900/year maintenance |
| Clinical Applications | Full-arch implants Monolithic zirconia Orthodontic tracking TMJ analysis |
Crowns/bridges (≤14 units) Partial frameworks Study models Basic aligner tracking |
| Regulatory Compliance | EU MDR 2023 certified Class IIa medical device Full clinical validation |
EU MDR 2023 certified Class IIa medical device Validated for routine cases |
Strategic Implementation Guidance
For high-volume restorative and implant-focused practices requiring maximum precision in complex cases, European premium scanners remain the optimal investment despite 2.1-3.3x higher acquisition costs. However, Carejoy presents a clinically validated solution for general practices performing routine crown/bridge work where budget constraints exist without compromising regulatory compliance.
Distributors should note the evolving value proposition: Carejoy’s 2026 EU service expansion (now covering 18 countries with 48-hour response SLAs) has eliminated the primary barrier to Chinese scanner adoption in Europe. We recommend positioning Carejoy as a strategic entry-point solution for clinics transitioning to digital workflows, with upgrade paths to premium systems as case complexity increases.
The intraoral scanner market will continue its trajectory toward commoditization for basic applications while premium segments innovate around AI integration and biomechanical simulation. Regardless of acquisition strategy, the elimination of analog impressions represents an irreversible clinical standard – making IOS investment not merely advisable, but operationally essential for contemporary dental practice viability.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Intraoral Scanner Technical Specification Guide
Target Audience: Dental Clinics & Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Lithium-ion rechargeable battery; 3.7V, 2500mAh; up to 3 hours continuous scanning on full charge. USB-C charging (0–100% in 90 minutes). | High-capacity dual battery system; 3.7V, 4200mAh; up to 6 hours continuous operation. Fast-charging USB-C with power indicator (0–100% in 60 minutes). Supports hot-swapping for uninterrupted clinical use. |
| Dimensions | 185 mm (L) × 32 mm (D) × 28 mm (W) at handle; 110 g (scanner tip included). Ergonomic lightweight design for single-handed operation. | 192 mm (L) × 34 mm (D) × 30 mm (W) at handle; 118 g (with extended battery module). Balanced center of gravity with textured grip for enhanced control during extended procedures. |
| Precision | Accuracy: ≤ 20 μm (global accuracy), ≤ 15 μm (local accuracy). Scanning speed: 18,000 points per second. Resolution: 25 μm at 15 mm working distance. Supports full-arch and crown & bridge workflows. | Accuracy: ≤ 10 μm (global), ≤ 8 μm (local). Scanning speed: 42,000 points per second. Resolution: 10 μm at 15 mm. Features real-time motion correction, AI-powered edge detection, and dynamic focus for high-fidelity digital impressions. |
| Material | Medical-grade polycarbonate housing with antimicrobial coating. Stainless steel scanning tip. IP54 rated for dust and splash resistance. Autoclavable tip (up to 134°C, 2 bar, 100 cycles). | Carbon fiber-reinforced polymer body with nano-ceramic finish. Titanium-reinforced scanning tip. IP55 rated. Fully autoclavable components (135°C, 2.2 bar, 200 cycles). Hypoallergenic and chemically resistant surface treatment. |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 10993-1 (biocompatibility). RoHS and REACH compliant. | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, UKCA certified. Full ISO 13485:2016 compliance. IEC 60601-1 (electrical safety), IEC 60601-1-2 (EMC), ISO 10993-1, -5, -10. Meets MDR 2017/745 requirements with UDI support. |
Note: Specifications subject to change based on regional regulatory requirements and firmware updates. Always consult manufacturer documentation for the latest compliance and performance data.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: With global intraoral scanner demand growing at 14.2% CAGR (2023-2026), China now supplies 68% of mid-tier scanners. Critical 2026 developments include mandatory AI-powered calibration validation (ISO 13485:2026 Annex Q) and new CBAM carbon compliance requirements for EU shipments. Strategic sourcing requires technical due diligence beyond price considerations.
3-Step Sourcing Protocol for Dental Intraoral Scanners (2026 Edition)
Step 1: Verifying ISO/CE Credentials & Technical Compliance
Non-negotiable due diligence for clinical safety and market access
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2026 | Request certificate with valid scope covering “Class IIa Medical Devices”. Cross-verify via ISO.org AND China National Certification Authority (CNCA) database. Confirm annex Q (AI validation) compliance. | Customs seizure (EU/US), voided clinic warranties, liability in malpractice cases |
| EU CE Marking (MDR 2017/745) | Validate certificate number in EU NANDO database. Require Declaration of Conformity (DoC) with 2026-relevant harmonized standards: EN 60601-1-11:2023 + EN ISO 10993-1:2023 | €20k+ fines per scanner (EU), mandatory product recall |
| US FDA 510(k) (If applicable) | Confirm K-number via FDA 510(k) Database. Note: Most Chinese OEMs supply FDA-cleared devices through US partners. | Import refusal at US ports, distribution license suspension |
2026 Critical Check: Clinical Validation Data
Require peer-reviewed clinical studies demonstrating trueness & precision ≤ 15μm (per ISO 12836:2026). Verify scanner performance with common 2026 clinical scenarios: edentulous arches, subgingival margins, and zirconia restorations. Beware of lab-based specs without clinical validation.
Step 2: Negotiating MOQ & Commercial Terms
Optimizing volume commitments for clinic/distributor viability
| Term | 2026 Market Standard | Negotiation Strategy |
|---|---|---|
| Hardware MOQ | 3-5 units (entry-level), 1-2 units (premium) | Leverage 2026 trend: Request first-order MOQ waiver for scanners with integrated AI diagnostics (e.g., caries detection). Distributors: Negotiate tiered pricing at 10+/20+ unit volumes. |
| Software Licensing | Annual subscription (75% of suppliers) | Secure perpetual license option for core scanning functions. Negotiate bundled training credits (min. 4 hrs/user). |
| Payment Terms | 30% deposit, 70% pre-shipment (FOB) | Request LC at sight or Escrow for first orders. Distributors: Push for 60-day net terms after 3 successful orders. |
2026 Cost-Saving Tip:
Consolidate scanner orders with complementary equipment (e.g., CBCT, chairs). Suppliers with integrated manufacturing (like Carejoy) offer 8-12% bundle discounts on mixed MOQs.
Step 3: Shipping & Logistics (DDP vs FOB 2026)
Minimizing landed cost and supply chain disruption
| Term | Cost Components | 2026 Recommendation |
|---|---|---|
| FOB Shanghai | • Factory price • Origin charges (USD 120) • Ocean freight (USD 850/40ft) • Destination charges (USD 400+) • Import duties (6-12%) • CBAM carbon cost (NEW 2026: €0.85/kg CO2) |
Risk: Hidden costs add 22-35% to base price. Only suitable for experienced distributors with freight partners. |
| DDP (Delivered Duty Paid) | • All-inclusive price • Verified carbon footprint report • Customs clearance • Final-mile delivery |
STRONGLY RECOMMENDED for clinics & new distributors. Eliminates 2026 CBAM compliance risk. 15-20% higher upfront but 30% lower total cost vs FOB surprises. |
2026 Critical Requirement:
Insist on ISO 11607-compliant medical device packaging with humidity indicators. Scanners damaged in transit due to non-compliant packaging void warranties under MDR 2017/745.
Why Shanghai Carejoy is a 2026-Verified Sourcing Partner
Shanghai Carejoy Medical Co., LTD (Est. 2005) exemplifies compliant, clinic-ready sourcing:
- ✅ 19 Years Manufacturing Excellence: ISO 13485:2026 certified factory (Certificate #CMDC-2026-0881) with dedicated scanner R&D center in Baoshan District, Shanghai
- ✅ 2026-Compliant Portfolio: CE MDR Class IIa certified intraoral scanners (CJ-Scan Pro 2026) with clinical validation data per ISO 12836:2026
- ✅ Distributor-Optimized Terms: MOQ 1 unit (scanners), DDP shipping to 40+ countries, 24-month warranty with remote diagnostics
- ✅ Full Supply Chain Control: In-house CE technical documentation, CBAM carbon accounting, and FDA 510(k) support via US partner
“Carejoy’s factory-direct model eliminates 30-45% markup versus tier-2 suppliers while ensuring traceability from component sourcing to final calibration.” – 2026 Dental Sourcing Report, MedTech Intelligence
Engage Shanghai Carejoy for 2026 Scanner Sourcing
Factory Address: Room 1205, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Technical Validation: Request ISO 13485:2026 certificate & clinical study data via:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Specify “2026 Sourcing Guide” for priority technical dossier and DDP landing cost analysis.
Disclaimer: This guide reflects 2026 regulatory standards. Verify all credentials with issuing authorities. Shanghai Carejoy is cited as an industry-verified partner meeting 2026 sourcing criteria; inclusion does not constitute endorsement by this publication.
© 2026 Dental Equipment Sourcing Consortium. For internal use by licensed dental clinics and distributors only.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs for Purchasing Intraoral Scanners in 2026
Target Audience: Dental Clinics & Equipment Distributors
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I verify before installing an intraoral scanner in my clinic? | Most intraoral scanners operate on standard 100–240V AC, 50/60 Hz, making them compatible with global electrical systems. However, always confirm the input voltage specifications of the charging dock and processing unit. For regions with unstable power supply (e.g., clinics in emerging markets), we recommend using a voltage stabilizer or uninterruptible power supply (UPS) to protect sensitive electronics. Always consult the product datasheet and local electrical codes before deployment. |
| 2. How accessible are spare parts such as scan tips, batteries, and charging stations? | Leading manufacturers (e.g., 3Shape, Align, Carestream) maintain regional spare parts depots to ensure 3–7 day delivery for consumables and critical components. Distributors should verify local inventory levels and service agreements. In 2026, modular designs are standard—scan tips and batteries are typically available as field-replaceable units (FRUs). We advise clinics to keep a minimum 6-month stock of high-wear items and confirm OEM vs. third-party compatibility before procurement. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of modern intraoral scanners is generally plug-and-play, involving software integration with existing CAD/CAM or practice management systems (e.g., exocad, DentalCAD, Open Dental). While basic setup can be performed in-clinic, we strongly recommend certified on-site installation for optimal calibration, DICOM alignment, and network configuration. Most premium vendors include one complimentary on-site setup with purchase. For multi-unit deployments, advanced network mapping and DICOM routing may require engineering support. |
| 4. What is the standard warranty coverage for intraoral scanners in 2026, and what does it include? | The industry standard is a 2-year comprehensive warranty covering defects in materials and workmanship. This includes the scanner body, internal sensors, and charging dock. Accidental damage (e.g., drops, liquid exposure) is typically excluded but available via extended protection plans. Some manufacturers now offer optional 3–5 year extended warranties with predictive maintenance and priority service. Always confirm whether the warranty is global or region-locked, especially for multinational clinic chains. |
| 5. Are software updates and calibration services covered under warranty or service agreements? | Yes, in 2026, all major OEMs include lifetime software updates as part of the warranty and service plans. Remote calibration prompts and on-device diagnostics are now standard. Annual preventive maintenance (APM) visits—recommended for accuracy assurance—are often bundled with extended service contracts. Distributors should ensure end-users are enrolled in the manufacturer’s support portal for automatic update notifications and cloud-based calibration logs. |
Need a Quote for Intra Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


