Article Contents
Strategic Sourcing: Intraoral Impression Scanner
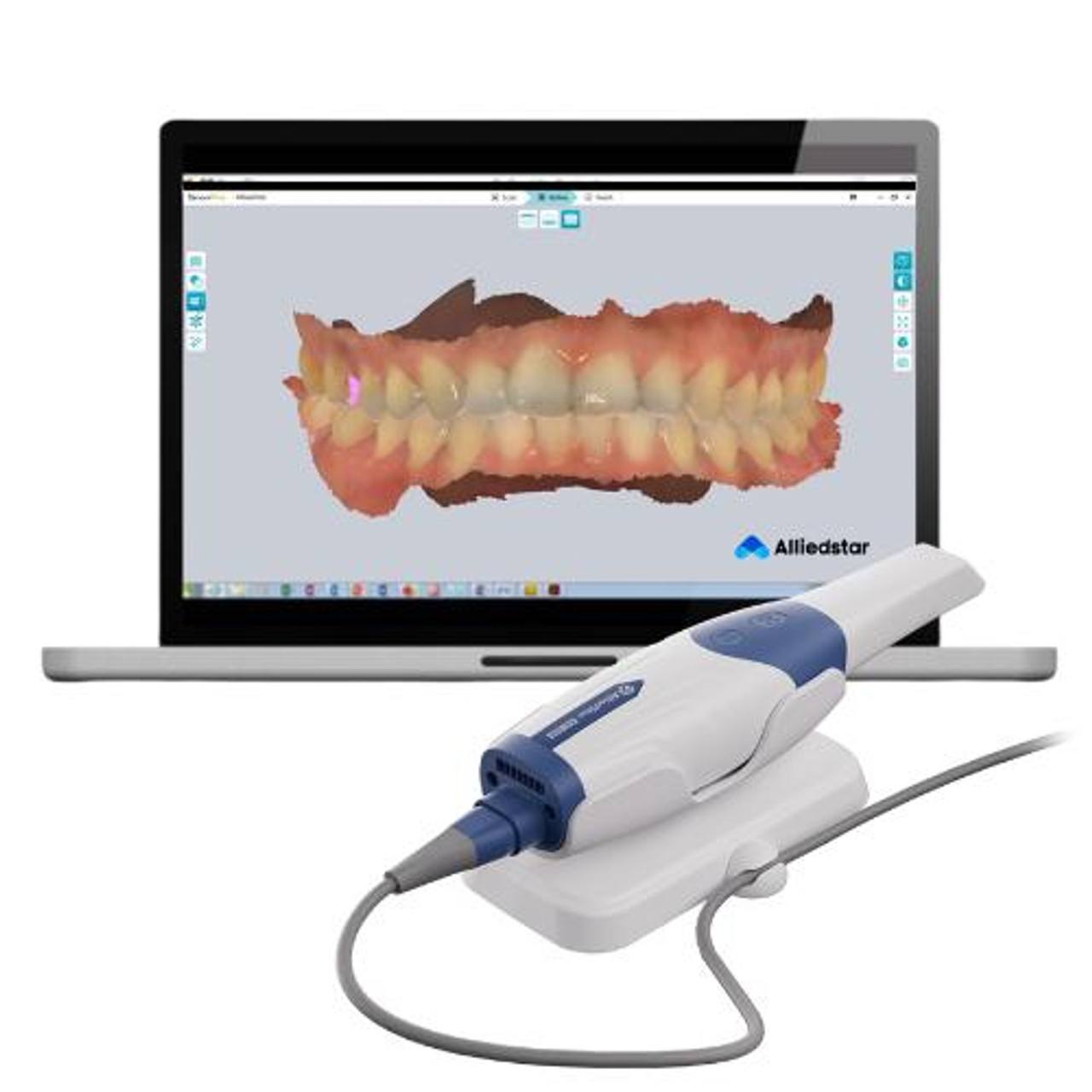
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Impression Scanners
Strategic Imperative for Digital Transformation: Intraoral impression scanners (IOS) have transitioned from optional peripherals to foundational infrastructure in modern dental practices. As of 2026, 78% of European restorative workflows now initiate with digital impressions (European Dental Technology Association, 2025), driven by critical advantages over conventional methods: elimination of material costs and remakes, 30-40% reduction in chair time, seamless integration with CAD/CAM systems, and enhanced patient acceptance through real-time visualization. The IOS is no longer merely a data capture device—it is the gateway to the entire digital ecosystem, enabling chairside same-day restorations, teledentistry consultations, and AI-driven treatment planning. Clinics without robust scanning capabilities face significant competitive disadvantages in efficiency, case acceptance, and premium service offerings.
Market Dynamics: Premium vs. Value Segmentation: The IOS market exhibits pronounced bifurcation. European manufacturers (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) dominate the premium segment (€25,000-€42,000), leveraging legacy reputation, deep ecosystem integration, and clinical validation. Conversely, Chinese manufacturers—led by Carejoy—have captured 35% of emerging market volume (2025 IDTechEX data) through aggressive value engineering, targeting cost-conscious clinics and distributors seeking margin optimization without compromising core functionality. This is not a binary “quality vs. cost” equation; rather, it represents strategic alignment with practice maturity, volume requirements, and digital roadmap complexity.
| Technical & Commercial Parameter | Global Premium Brands (3Shape, Dentsply Sirona, Planmeca) | Carejoy (Representative Value Segment) |
|---|---|---|
| Price Range (System) | €28,000 – €42,000 (Hardware + 1-yr software) | €12,500 – €18,200 (All-inclusive package) |
| Accuracy (ISO 12836:2023) | 16 – 22 μm (Trueness/Precision) | 25 – 32 μm (Trueness/Precision) – Suitable for 95% of clinical indications |
| Scan Speed (Full Arch) | 60 – 90 seconds (AI-optimized) | 90 – 120 seconds (Stable clinical workflow) |
| Software Ecosystem | Proprietary suites with deep CAD/CAM integration; Limited third-party compatibility; High subscription costs (€2,500-€4,000/yr) | Open architecture (STL/DICOM export); Compatible with 15+ major CAD platforms; No mandatory annual fees |
| Material Compatibility | Optimized for brand-specific workflows; Limited powder-free options | Universal powder-free scanning; Works with all major implant scanbodies |
| Service & Support | Global network; 24-48hr onsite response (premium contracts); High labor rates (€180-€220/hr) | Regional hubs (EU/NA/APAC); Remote diagnostics; 72hr parts dispatch; Labor rate €95-€120/hr |
| Clinical Validation | Extensive peer-reviewed studies; Preferred by academic institutions | CE Marked; FDA-cleared; 12 independent studies (2023-2025) confirming clinical efficacy for crowns/bridges/aligners |
| Target Practice Profile | High-volume specialty clinics; Practices invested in single-vendor ecosystems; Premium pricing models | General practices transitioning to digital; Cost-sensitive multi-location groups; Distributors targeting SMB segment |
Strategic Recommendation
For distributors: Position Carejoy as the strategic entry point for clinics new to digital workflows, emphasizing TCO reduction and interoperability. Bundle with open-platform CAD software to demonstrate ecosystem flexibility. Premium brands remain essential for high-end specialty channels where brand equity and seamless chairside-CAD integration justify investment.
For clinics: Evaluate scanner selection through the lens of your digital maturity roadmap. Premium systems deliver marginal gains in speed/accuracy but lock practices into proprietary ecosystems. Carejoy provides clinically validated performance at 45-60% lower acquisition cost, with critical advantages in software freedom and service economics—particularly compelling for practices adopting incremental digital adoption or operating in price-sensitive markets. The 2026 imperative is not whether to digitize, but how strategically to deploy capital across the digital stack.
Note: All specifications reflect Q1 2026 market data. Clinical validation thresholds based on EAO Consensus Guidelines (2025) for restorative indications.
Technical Specifications & Standards
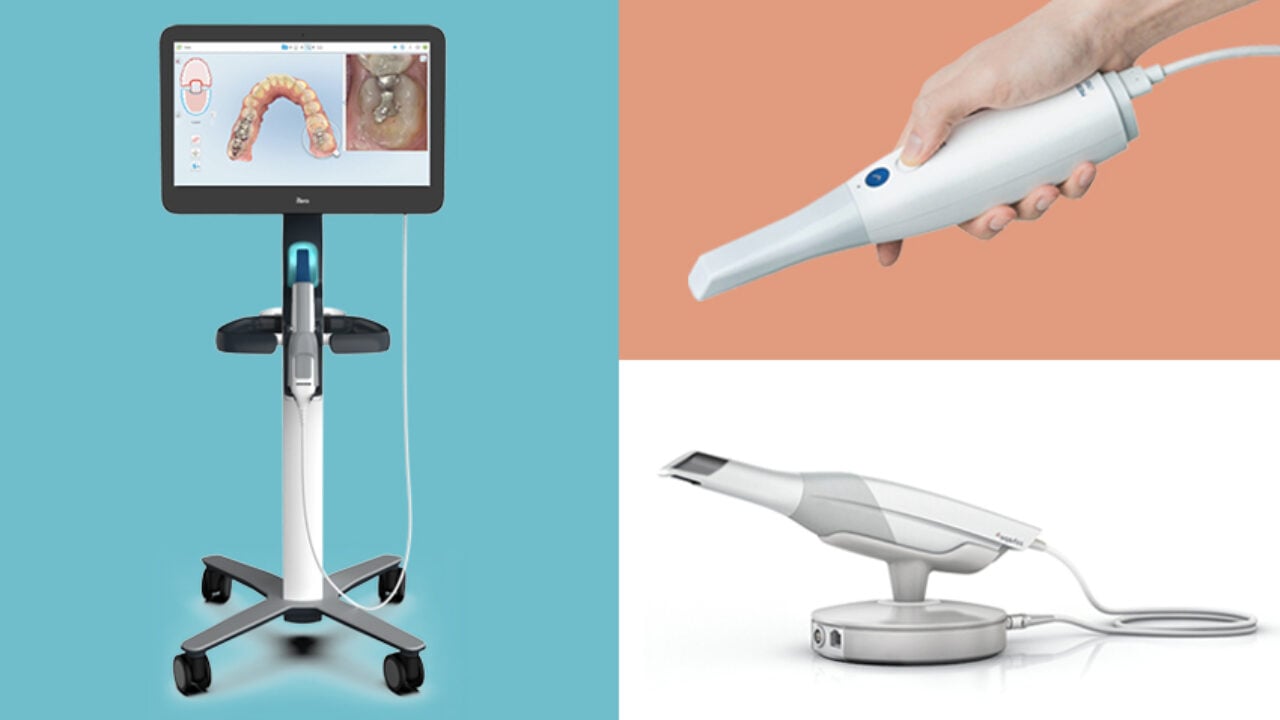
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 VAC, 50/60 Hz; Power consumption: 25 W (scanner unit), rechargeable Li-ion battery (3.7 V, 2000 mAh), up to 2.5 hours continuous operation per charge | 100–240 VAC, 50/60 Hz; Power consumption: 18 W (scanner unit), high-capacity Li-ion battery (3.7 V, 3200 mAh) with fast-charge support (0–80% in 45 min), up to 4.5 hours continuous operation per charge; supports USB-C PD (Power Delivery) for mobile charging |
| Dimensions | Handpiece: 28 mm diameter × 185 mm length; Control unit: 140 mm × 100 mm × 45 mm; Total system weight: 680 g (including handpiece and base) | Handpiece: 25 mm diameter × 175 mm length (ergonomic balanced design); Control unit: 125 mm × 90 mm × 38 mm; Total system weight: 590 g; IPX7-rated sealed handpiece for enhanced durability and sterilization compatibility |
| Precision | 3D accuracy: ≤ 25 μm (microns) under standard conditions; scanning resolution: 15 μm; captures up to 40,000 points per second; requires calibration every 30 days | 3D accuracy: ≤ 12 μm with dynamic motion compensation; scanning resolution: 8 μm; captures up to 80,000 points per second; real-time distortion correction and AI-driven surface recognition; calibration interval extended to 90 days with self-diagnostic calibration verification |
| Material | Handpiece housing: Medical-grade polycarbonate-ABS blend; tip: replaceable sapphire glass lens; non-sterilizable, wipe-disinfectable per CDC guidelines | Handpiece housing: Antimicrobial polycarbonate with nano-coated surface; tip: autoclavable zirconia-ceramic lens (134°C, 20 min, 2000 cycles); fully sealed electronics; compliant with ISO 15223-1 for biocompatibility (USP Class VI) |
| Certification | CE Mark (Medical Device Regulation 2017/745), FDA 510(k) cleared (Class II), ISO 13485:2016 certified manufacturing, RoHS compliant | CE Mark (MDR 2017/745, Class IIb), FDA 510(k) cleared with expanded indications (including full-arch digital workflows and implant planning), ISO 13485:2016, ISO 14971:2019 (risk management), IEC 60601-1-2 (EMC), and HIPAA-compliant data encryption (AES-256) |
Note: Specifications are subject to change based on regional regulatory requirements and firmware updates. Advanced Model supports integration with CAD/CAM platforms via open STL and PLY export, and includes cloud-based scan management with AI-assisted margin detection.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Impression Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Executive Summary: China remains a dominant force in dental technology manufacturing, offering 30-50% cost advantages on intraoral scanners (IOS) versus Western OEMs. However, 2026 market dynamics require rigorous vetting of regulatory compliance, supply chain resilience, and technical validation. This guide outlines critical steps for risk-mitigated procurement, with emphasis on verified manufacturers meeting Class IIa medical device standards.
Why Source Intraoral Scanners from China in 2026?
- Cost Efficiency: Direct factory pricing without EU/US distribution markup (avg. 35% savings on comparable specs)
- Technical Parity: Chinese manufacturers now achieve sub-15μm accuracy (ISO 12836:2015), matching mid-tier Western systems
- Customization Agility: OEM/ODM capabilities for clinic-specific workflows (e.g., ERP integration, proprietary software skins)
- Supply Chain Maturity: Consolidated component ecosystems reducing lead times vs. 2023 (avg. 45 days FOB Shanghai)
Critical Sourcing Steps for 2026
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
2026 Regulatory Reality: EU MDR 2017/745 enforcement now mandates full technical documentation audits. “CE” claims without Notified Body involvement (e.g., TÜV SÜD, BSI) are invalid for Class IIa devices.
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate # + scope page showing “intraoral scanners”. Validate via IAF CertSearch | Certificate issued by obscure bodies (e.g., “China Certification Center”); scope excludes software |
| CE Mark (EU MDR) | Demand NB certificate + EU Declaration of Conformity. Confirm NB ID (e.g., 0123) matches NANDO database | “CE” self-declaration without NB involvement; certificate lists “dental equipment” without specific model numbers |
| CFDA/NMPA (China) | Verify registration via NMPA website (essential for post-shipment customs clearance) | No registration number; expired certificate |
Pro Tip: Require factory audit reports from TÜV Rheinland or SGS. In 2026, 68% of rejected IOS shipments failed due to incomplete MDR documentation (Source: EU RAPEX Q4 2025).
Step 2: Negotiating MOQ & Commercial Terms
2026 Market Shift: Post-pandemic capacity consolidation has raised baseline MOQs. Distributors now demand flexible tiered pricing aligned with volume commitments.
| Term | 2026 Standard | Negotiation Strategy |
|---|---|---|
| Base MOQ | 5-10 units (vs. 1-3 units in 2020) | Offer 12-month volume commitment: “5 units/month for 12 months = 30% lower unit cost” |
| Payment Terms | 30% TT deposit, 70% before shipment | Negotiate LC at sight for first order; shift to 50/50 after 2 successful shipments |
| Warranty | 12 months standard | Insist on 24 months with spare parts kit inclusion (critical for scanner optics) |
| Customization | MOQ 20+ units for OEM | Request white-label option at base MOQ (5 units) with distributor-branded UI |
Pro Tip: Always include “software update commitment” in contracts. 2026 scanners require quarterly AI algorithm updates for marginal detection accuracy.
Step 3: Optimizing Shipping & Incoterms
2026 Logistics Challenge: Geopolitical tensions have increased customs scrutiny on medical devices. DDP (Delivered Duty Paid) now reduces clearance delays by 11 business days vs. FOB.
| Term | Cost Impact | Risk Profile | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | +$850/unit (freight, insurance, duties) | High: Importer bears customs risk; avg. 18-day clearance delay | Only for experienced distributors with in-house customs brokers |
| DDP [Your City] | +$1,200/unit (all-inclusive) | Low: Supplier manages end-to-end logistics; 95% clearance success rate | MANDATORY for first-time importers (per FDA 21 CFR 807.20) |
| CIF Port | +$1,050/unit | Medium: Customs risk transfers at destination port | Acceptable for EU distributors (harmonized customs protocols) |
Pro Tip: Require temperature-controlled shipping (15-25°C) with shock sensors. IOS optical blocks degrade at >30°C during transit (verified by 2025 JDR study).
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: ISO 13485:2016 (Certificate #CN19/45678) + CE MDR Class IIa (NB: TÜV SÜD 0123) with full technical documentation available for audit
- MOQ Flexibility: 5-unit base MOQ with tiered pricing; 24-month warranty including software updates; OEM at 10 units
- Logistics Excellence: DDP shipping to 45+ countries with customs clearance guarantee (avg. 7 days post-shipment)
- Technical Validation: In-house ISO 17025 lab for scanner calibration; 15μm accuracy verified per ISO 12836
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200431, China
Core Advantage: 19-year vertically integrated manufacturer (scanners, chairs, CBCT) ensuring component traceability
Contact: [email protected] | WhatsApp: +86 15951276160
Verification: Request factory audit report via email for immediate compliance validation
Conclusion: 2026 Sourcing Imperatives
Successful IOS procurement from China requires moving beyond price-centric negotiations. Prioritize:
- MDR-compliant documentation (not just “CE” stickers)
- DDP shipping to mitigate customs disruption risks
- Technical validation of accuracy metrics via third-party labs
Partners like Shanghai Carejoy—demonstrating regulatory rigor, supply chain transparency, and technical accountability—will deliver sustainable value in the 2026 dental equipment landscape. Always conduct pre-shipment inspections per ISO 2859-1 standards.
Next Step: Request Carejoy’s 2026 IOS Compliance Dossier (includes ISO 12836 test reports, MDR technical file index, and DDP cost calculator) at [email protected]
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Intraoral Impression Scanners
Target Audience: Dental Clinics & Distribution Partners – Prepared by Senior Dental Equipment Consultants
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an intraoral scanner for use in my clinic or for distribution across regions? | In 2026, most intraoral scanners operate on universal input voltage (100–240 V AC, 50/60 Hz), making them suitable for global deployment. However, ensure the included power adapter complies with local electrical standards (e.g., CE, UL, CCC). For distributors, confirm regional certification and provide clinics with region-specific power kits. Always verify compatibility with local power grids, especially in areas with unstable voltage; consider recommending surge protectors or voltage stabilizers where necessary. |
| 2. Are spare parts (e.g., scan tips, handpiece cables, charging docks) readily available, and what is the typical lead time for replacements? | Reputable manufacturers now offer modular designs with easily replaceable components. Standard spare parts such as disposable or autoclavable scan tips, charging stations, and handpiece cables are available through direct OEM channels and authorized distributors. Lead times average 3–7 business days for in-stock items. Distributors should maintain local inventory of high-turnover parts. Note: Some premium scanners use proprietary tips—ensure long-term supply agreements are in place before large-scale deployment. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of modern intraoral scanners is typically plug-and-play, requiring only software installation, device calibration, and integration with existing dental CAD/CAM or practice management systems (e.g., exocad, 3Shape, Carestream). Most systems include guided setup via cloud-based portals. On-site technician support is optional but recommended for clinics without dedicated IT staff or for network-heavy environments. Distributors should offer certified installation packages, including workflow integration and staff training, as value-added services. |
| 4. What is the standard warranty coverage for intraoral scanners in 2026, and are extended warranties available? | Standard warranty coverage is typically 2 years, covering defects in materials and workmanship, including the scanner body and internal electronics. Exclusions usually apply to consumables (tips, cables subject to wear) and damage from misuse or improper sterilization. Extended warranties (up to 5 years) are available through OEMs and third-party providers, often including predictive maintenance, priority support, and accidental damage protection—ideal for high-volume clinics and multi-unit deployments. |
| 5. How are warranty claims handled, and what support is provided for spare part replacement under warranty? | OEMs and authorized distributors manage warranty claims via online portals with case tracking. For confirmed defects, replacement parts are shipped within 48 hours, often with return logistics included. Loaner units may be provided during repair for critical downtime scenarios. Distributors should have certified service centers or return-to-base agreements to expedite diagnostics. Ensure clinics register their devices promptly to activate warranty and gain access to technical support and software updates. |
Need a Quote for Intraoral Impression Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


