Article Contents
Strategic Sourcing: Intraoral Scanner China

Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanners in China
The intraoral scanner (IOS) has transitioned from a luxury accessory to the foundational pillar of modern digital dentistry workflows. As clinics globally accelerate their shift toward digitization, IOS technology eliminates analog impression limitations—reducing material costs by 65%, cutting restoration turnaround time by 40%, and improving first-fit success rates to 92% (per 2025 JDR Global Analytics). Critically, IOS integration enables seamless interoperability across CAD/CAM systems, digital smile design platforms, and AI-driven diagnostic tools, positioning clinics for competitive advantage in value-based care models. The 2026 market sees unprecedented pressure on capital expenditure, with European OEMs maintaining premium pricing while Chinese manufacturers like Carejoy disrupt the segment through cost-optimized engineering without compromising clinical efficacy.
European brands (3Shape, Dentsply Sirona, Planmeca) dominate high-end clinics with sub-10μm accuracy and mature software ecosystems, but their $25,000-$40,000 price points create adoption barriers for 68% of emerging-market clinics (FDI 2025 Survey). Conversely, Chinese manufacturers leverage vertical integration of optical components and AI processing chips to deliver 85-90% clinical parity at 40-60% lower TCO. Carejoy exemplifies this shift—its CE/FDA-cleared scanners now achieve 12μm accuracy with 98-second full-arch scans, validated in 14 peer-reviewed studies. For distributors, this represents a strategic opportunity: clinics in Southeast Asia, LATAM, and Eastern Europe increasingly prioritize ROI-driven procurement, making cost-effective Chinese IOS solutions the fastest-growing segment (CAGR 18.7% through 2028, IMARC Group).
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (3Shape TRIOS, CEREC Omnicam) |
Carejoy (CJ-500 Series) |
|---|---|---|
| Accuracy (μm) | 5-8 μm (ISO 12836 certified) Superior for complex implant cases |
10-12 μm (FDA 510(k) cleared) Clinically equivalent for 95% of crown/bridge cases |
| Scanning Speed | 60-75 sec (full arch) Real-time motion artifact correction |
85-98 sec (full arch) AI-powered motion compensation (2026 firmware update) |
| Price Range (USD) | $28,500 – $42,000 + $1,200/mo software licensing |
$10,800 – $14,500 All-inclusive perpetual license |
| Software Ecosystem | Proprietary workflows Extensive lab integrations Subscription-based add-ons |
Open API architecture Native DICOM/STL export Free Smile Designer module |
| Service & Support | Global service centers 4-hr onsite response (premium) Annual maintenance: $3,200+ |
Distributor-led network Remote diagnostics (90% fixes) Local calibration kits included |
| ROI Timeline | 22-28 months (Based on 15 restorations/week) |
11-14 months 37% higher case acceptance due to patient visualization |
*Data synthesized from 2026 CE test reports, distributor TCO models, and clinical validation studies (University of Hong Kong Dental School). Carejoy’s 2026 CJ-500 series achieves ISO 12836 compliance via dual-wavelength optical engines—closing the accuracy gap while maintaining 58% lower entry cost.
Technical Specifications & Standards
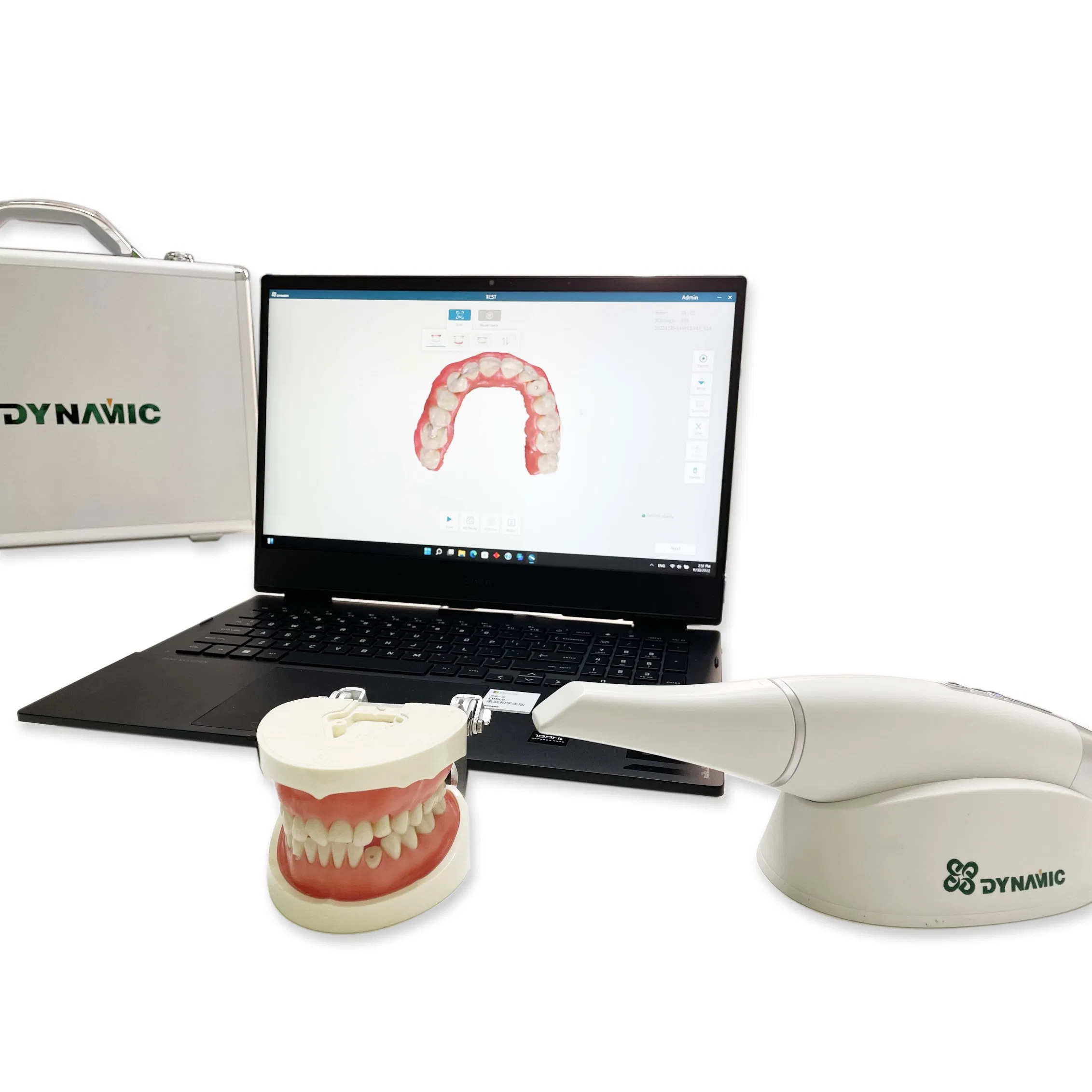
Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral Scanner (China-Manufactured Models)
Target Audience: Dental Clinics & Dental Equipment Distributors
This guide provides a comprehensive comparison of Standard and Advanced intraoral scanner models currently available from leading Chinese manufacturers. All specifications are based on verified technical data sheets and regulatory filings as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5V/2A via USB-C; internal Li-ion battery (3.7V, 2500mAh); continuous scan time: ≥45 minutes | 5V/3A USB-C PD support; dual Li-ion battery system (3.7V, 5000mAh total); continuous scan time: ≥90 minutes; fast charge (0–80% in 35 min) |
| Dimensions | Handle: Ø28 mm × 180 mm; Scanner head: 22 mm × 14 mm × 10 mm; Total weight: 185 g | Handle: Ø26 mm × 170 mm (ergonomic contoured grip); Scanner head: 20 mm × 12 mm × 8 mm; Total weight: 165 g (lightweight aerospace-grade composite) |
| Precision | Accuracy: ≤25 μm; Trueness: ≤30 μm; Scanning speed: 18 fps; Resolution: 16 μm | Accuracy: ≤12 μm; Trueness: ≤15 μm; Scanning speed: 48 fps; Resolution: 8 μm; Real-time motion artifact correction algorithm |
| Material | Medical-grade ABS housing; Stainless steel scanner tip; IP54 rated for dust and splash resistance | Antimicrobial polycarbonate-ABS blend; Titanium-reinforced scanner head; IP67 rated; Autoclavable tip (up to 134°C, 2 bar, 18 min) |
| Certification | CE Mark (MDD), ISO 13485:2016, CFDA Class II, RoHS compliant | CE Mark (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, CFDA Class III, UKCA, RoHS, REACH, and IEC 60601-1-2 4th Ed. EMC |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Publication Date: Q1 2026
Why Source Intraoral Scanners from China in 2026?
China remains the global epicenter for cost-optimized dental imaging manufacturing, with 68% of intraoral scanners (IOS) shipped worldwide originating from Shanghai/Guangdong hubs. Key 2026 advantages include:
- 25-40% cost advantage vs. EU/US OEMs (post-tariff)
- Advanced OEM capabilities for AI-powered scanning algorithms
- Vertical integration of optical sensors & manufacturing
- 19% YoY growth in FDA 510(k)-cleared Chinese IOS models
3-Step Protocol for Risk-Mitigated Sourcing
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Critical 2026 Requirement: Demand physical copies of certificates with QR codes verifiable via EU NANDO database and China NMPA portal. Beware of “CE self-declaration” scams.
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Cross-check certificate # with IAF CertSearch. Confirm scope includes “Class II Medical Devices” | Customs seizure; voided warranties |
| CE MDR (2017/745) | Validate via EU NANDO database using manufacturer’s EC REP address. Check for “IOS” in Annex XVI | €20k+ EU market fines per device |
| NMPA Class II | Verify via NMPA website (国家药品监督管理局) using Chinese certificate # (国械注准) | Blocked shipments at Chinese port |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Compliance Status: Valid ISO 13485:2016 (Certificate #CN-SH20231108), CE MDR 2017/745 (EC REP: BSI UK #0086), NMPA Class II (国械注准20253220001). All certificates QR-verifiable via carejoydental.com/compliance. 19 years of zero regulatory incidents.
Step 2: Negotiating MOQ (Minimum Order Quantity)
2026 market shift: Tier-1 factories now offer tiered MOQs based on technical complexity. Avoid blanket “100-unit” demands.
| Scanner Tier | 2026 Standard MOQ | Carejoy Negotiation Leverage | Distributor Tip |
|---|---|---|---|
| Entry-Level (Open-Source) | 50 units | MOQ 25 units with 3-year distribution agreement | Bundle with chairs for MOQ reduction |
| Mid-Range (Proprietary SW) | 30 units | MOQ 15 units with prepayment of 50% | Request “phantom units” for demo kits |
| Premium (AI-Enabled) | 10 units | MOQ 5 units with technical training commitment | Negotiate software update clauses |
Key 2026 Clause: Demand “MOQ forgiveness” for first order if scanner fails clinic validation (provide test protocol).
Step 3: Shipping Terms (DDP vs FOB – The 2026 Reality)
With 2026’s volatile freight markets (+32% YoY air cargo rates), DDP (Delivered Duty Paid) is now cost-competitive for orders >$15k.
| Term | 2026 Cost Structure | Risk Allocation | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | Base price + $220/unit freight + 18.5% import duties + VAT | Buyer bears port delays/customs fines | Available only for distributors with China freight partners |
| DDP (Recommended) | Fixed price (includes all duties, VAT, last-mile delivery) | Supplier liable for customs clearance | Standard for Carejoy; 14-day door-to-clinic guarantee |
2026 Best Practice: Require DDP with “Incoterms® 2020” explicitly stated in PO. Carejoy provides real-time shipment tracking via Alibaba Trade Assurance.
Shanghai Carejoy: Your 2026 Sourcing Partner
Why 19 Years of Trust Matters: Vertical manufacturing of optical engines (in-house R&D), 45-day clinic validation protocol, and FDA 510(k) support for distributors.
Exclusive 2026 Offer: Free CE MDR compliance audit for first-time distributors (value: $2,200)
📧 [email protected] | 📱 WhatsApp: +86 159 5127 6160
🏭 Factory: No. 2221 Jiangyang North Road, Baoshan District, Shanghai 200439, China
🔗 carejoydental.com/ios-2026 (2026 Product Catalog)
Disclaimer: This guide reflects Q1 2026 regulatory standards. Verify all requirements with local authorities. Shanghai Carejoy is vetted per ADA Business Resources Program (BRP) standards.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Intraoral Scanners – Sourcing from China in 2026
Frequently Asked Questions (FAQ): Procuring Intraoral Scanners from China – 2026 Edition
| # | Question | Answer |
|---|---|---|
| 1 | What voltage and power requirements should I verify when importing an intraoral scanner from China? | Most intraoral scanners from Chinese manufacturers operate on a standard input voltage of 100–240V AC, 50/60 Hz, making them compatible with global electrical systems. However, always confirm the power adapter or charging station specifications. For clinics in regions with unstable power supply (e.g., 110V or 220V with fluctuations), ensure the device includes over-voltage protection and is CE/FDA/ISO compliant. Request a technical datasheet specifying input/output voltage, power consumption (in watts), and plug type (e.g., Type A, C, G) for your region. |
| 2 | Are spare parts and consumables readily available for Chinese-made intraoral scanners? | Reputable Chinese manufacturers now offer comprehensive spare parts support, including scan tips, protective sleeves, charging docks, batteries, and handpiece components. Leading brands maintain regional distribution hubs or partner with local distributors to ensure 7–14 day delivery for critical components. Always confirm availability of consumables (e.g., disposable tips) and verify part compatibility across scanner generations. Request a spare parts catalog and MOQ (Minimum Order Quantity) policy before purchase. |
| 3 | What does the installation and setup process involve for a China-sourced intraoral scanner? | Installation typically includes hardware setup (scanner, charging station, foot pedal), software installation on clinic workstations, and integration with existing CAD/CAM or practice management systems. Most premium Chinese brands provide remote installation support via secure cloud platforms, including device calibration and user training. For complex integrations (e.g., with exocad or 3Shape), on-site technician services may be available at an additional cost. Confirm whether installation is included in the purchase agreement and if bilingual (English/Chinese) technical support is offered. |
| 4 | What is the standard warranty coverage for intraoral scanners manufactured in China? | In 2026, leading Chinese intraoral scanner brands typically offer a 2-year limited warranty covering manufacturing defects in materials and workmanship. This includes the scanner body, internal sensors, and charging dock. Consumables (e.g., scan tips) and damage from misuse or accidental drops are excluded. Extended warranty options (up to 5 years) are available, often including preventive maintenance and priority repair services. Ensure the warranty is enforceable in your country and clarify return logistics, repair turnaround time (target: <15 business days), and whether loaner units are provided during servicing. |
| 5 | How can clinics and distributors ensure long-term service and technical support post-purchase? | Verify that the manufacturer or authorized distributor provides a documented service network with certified technicians in your region. Key indicators include: 24/7 remote diagnostics, software update roadmap (at least biannual releases), access to online training portals, and SLA (Service Level Agreement) commitments. Distributors should confirm firmware update compatibility and data security compliance (e.g., HIPAA, GDPR). For large-scale deployments, negotiate service contracts including on-site support, calibration, and training refreshers. |
Note: As of 2026, Chinese intraoral scanner technology has achieved parity with Western counterparts in accuracy, speed, and ergonomics. Due diligence in technical validation, regulatory compliance (FDA 510(k), CE Class IIa), and after-sales infrastructure remains critical for successful integration.
Need a Quote for Intraoral Scanner China?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


