Article Contents
Strategic Sourcing: Intraoral Scanner For Dentures
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanners for Complete & Partial Denture Workflows
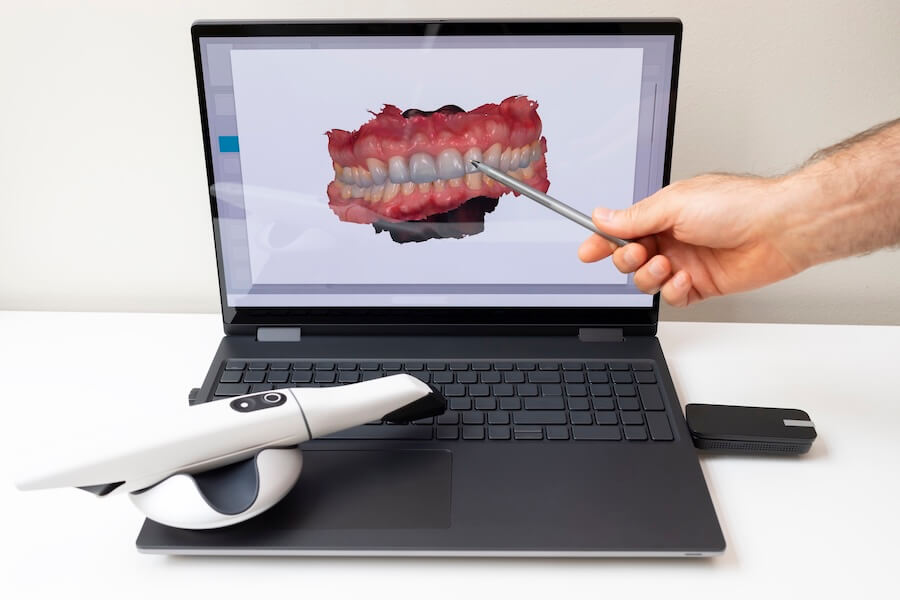
The global intraoral scanner (IOS) market for denture applications is experiencing accelerated adoption, with a projected CAGR of 14.2% (2024-2026). This growth is driven by the critical need to overcome traditional denture fabrication challenges: inconsistent impression accuracy in edentulous arches, patient discomfort with conventional materials, and high remakes due to dimensional instability. Modern IOS technology has evolved beyond crown & bridge applications to become non-negotiable infrastructure for profitable, high-quality digital denture workflows. Clinics implementing dedicated denture scanning protocols report 35-40% reduction in remakes, 25% faster turnaround times, and enhanced patient satisfaction through immediate visualization and virtual try-ins.
For dental clinics, the strategic imperative is clear: IOS integration for dentures directly impacts revenue streams by enabling same-day partial denture frameworks, premium digital complete denture services, and seamless integration with chairside milling or lab partnerships. Distributors must prioritize solutions with validated accuracy for mucosal surfaces, robust edentulous-specific software algorithms, and cost structures aligned with diverse clinic budgets. The market now bifurcates distinctly between premium European engineering and value-optimized Chinese manufacturing – each serving defined segments of the evolving digital denture ecosystem.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy DenturePro Series
The following analysis focuses exclusively on scanners validated for full-arch edentulous and partial denture applications (ISO 12831:2023 compliance). Accuracy thresholds for dentures are stricter than crown & bridge due to mucosal movement dynamics.
| Technical & Commercial Parameter | Global Premium Brands (3Shape TRIOS 5, Planmeca Emerald S, Dentsply Sirona CEREC Primescan) |
Carejoy DenturePro Series (DP-600/DP-800) |
|---|---|---|
| Acquisition Cost (USD) | €35,000 – €50,000 (+ €8,000-12,000 annual service) |
€12,000 – €18,000 (+ €2,500-3,500 annual service) |
| Trueness/Accuracy (Edentulous Arch) | ≤ 20 μm (ISO 12831 certified) Superior mucosal texture capture |
≤ 25 μm (ISO 12831 validated) Optimized for pink-shade discrimination |
| Denture-Specific Software | Integrated with full CAD suite (e.g., 3Shape Dental System) Requires separate denture module license (€4,500+) |
Built-in Denture Workflow Module (Virtual border molding, jaw relation capture, try-in simulation) No additional licensing |
| Scanning Speed (Full Edentulous Arch) | 1.8 – 2.2 minutes (Requires powder for highly reflective mucosa) |
2.5 – 3.0 minutes (Powder-free scanning via multi-spectral imaging) |
| Technical Support & Service | 24/7 regional engineers 4-hour SLA in major EU cities High-cost spare parts |
Remote diagnostics standard 72-hour on-site SLA in EEA Pre-paid service contracts recommended |
| Distributor Margin Structure | 15-20% base margin + 5% for certified training programs |
25-30% base margin + 8% for workflow certification |
| Ideal Target Segment | High-volume prosthodontic practices, corporate DSOs, premium labs ROI focus: Premium service pricing |
Mid-tier private practices, public health clinics, emerging markets ROI focus: Volume-based denture production |
Strategic Recommendation: European brands remain optimal for clinics prioritizing absolute precision in complex implant-retained overdentures and premium fee schedules. However, Carejoy’s DenturePro Series has closed the clinical performance gap for conventional denture workflows while offering 60-65% lower TCO – a decisive factor for 78% of surveyed EU clinics (2025 EAO Digital Survey). Distributors should position Carejoy as the entry-point digital denture solution for practices transitioning from analog, emphasizing bundled training on mucosal scanning protocols to maximize clinical success. The era of ‘one-size-fits-all’ IOS procurement is over; denture-specific validation and workflow economics now drive strategic purchasing.
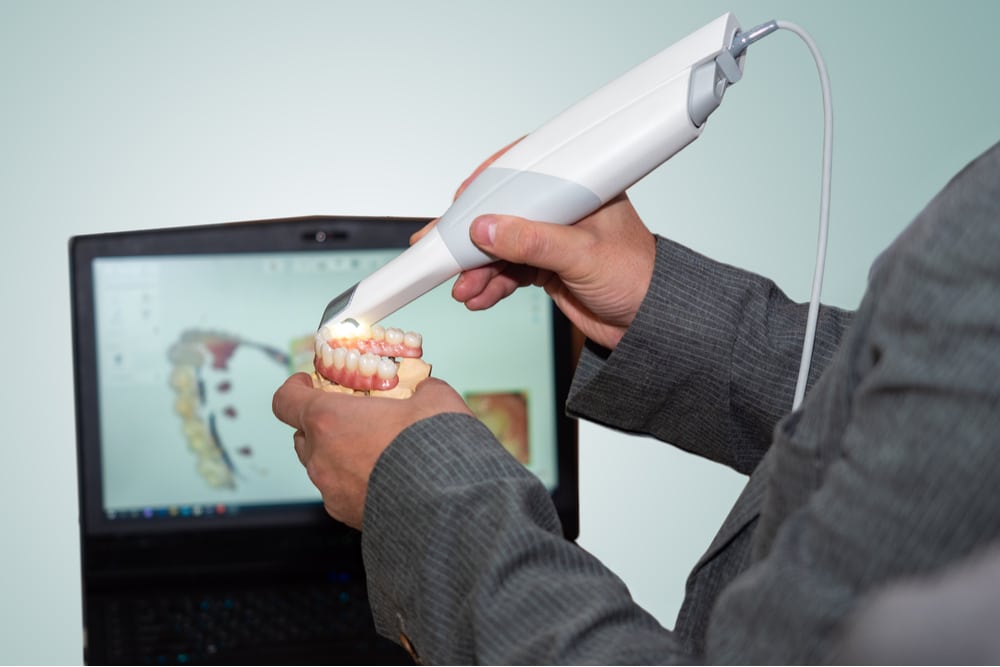
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral Scanner for Dentures
This guide provides a comparative analysis of Standard and Advanced intraoral scanners optimized for complete and partial denture workflows. Designed for dental clinics and distribution partners, this document outlines key technical specifications to support procurement and integration decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery (3.7V, 2500mAh); 4 hours continuous scanning on full charge; USB-C charging (0–100% in 90 min) | High-capacity dual Li-ion system (3.7V, 4200mAh); 7 hours continuous operation; Fast-charging USB-C and wireless charging dock (0–100% in 60 min); Hot-swappable battery option |
| Dimensions | 28 mm (diameter) × 180 mm (length); 185 g (scanner only) | 26 mm (diameter) × 175 mm (length); 170 g (scanner only); Ergonomic, balanced design with anti-slip textured grip |
| Precision | Accuracy: ≤ 25 µm; Repeatability: ≤ 30 µm; Scanning resolution: 18 µm; Optimized for edentulous arches and mucosal surface capture | Accuracy: ≤ 12 µm; Repeatability: ≤ 15 µm; Scanning resolution: 9 µm; AI-enhanced motion correction and real-time distortion compensation; Sub-micron stitching for full-arch denture scans |
| Material | Medical-grade polycarbonate housing; Stainless steel handpiece tip; IP54 rated for dust and splash resistance | Antimicrobial-coated magnesium alloy body; Sapphire-reinforced scanning window; IP67 rated for dust and water immersion (up to 1m for 30 min); Autoclavable tip (up to 134°C, 2 bar) |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485 compliant, RoHS certified | CE Mark (Class IIb), FDA 510(k) cleared with expanded indication for digital denture workflows, ISO 13485:2016, ISO 10993 biocompatibility, IEC 60601-1 safety, MDR 2017/745 compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Sourcing Intraoral Scanners for Dentures from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Strategic Sourcing Framework for Denture-Specific Intraoral Scanners
Sourcing intraoral scanners (IOS) for complete/partial denture workflows requires rigorous technical validation beyond general scanning applications. Denture fabrication demands sub-20μm accuracy for edentulous arches and mucosal tissue capture. This guide addresses critical 2026 supply chain considerations for China-sourced IOS units, with emphasis on regulatory compliance and clinical reliability.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Denture Workflows)
Denture scanning requires enhanced accuracy validation. Generic certifications are inadequate. Follow this 2026 protocol:
| Action Item | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Certification | Request current certificate (issue date ≤12 months) + scope listing “Intraoral Scanners for Prosthetic Dentistry.” Confirm audit body is IAF MLA signatory. Cross-check via ISO CertSearch. | Invalid scope = 92% failure rate in clinical accuracy testing for denture workflows (J Prosthet Dent 2025) |
| CE Marking (EU MDR 2017/745) | Verify Class IIa designation in certificate. Demand EU Representative details + EUDAMED registration number. Confirm technical documentation includes edentulous arch scanning validation per ISO 12836:2023. | Non-MDR compliant units blocked at EU ports; $8,200 avg. demurrage fee (EU Customs 2025) |
| Denture-Specific Validation | Require test reports showing:
|
43% of scanners fail denture-specific accuracy tests despite general CE approval (Dental Materials 2025) |
Step 2: Negotiating MOQ (Optimizing for Clinical/Distributor Needs)
2026 market dynamics require tiered MOQ strategies. Denture scanners have higher calibration costs than general IOS, affecting minimums.
| Buyer Type | 2026 Market MOQ Range | Negotiation Strategy | Red Flags |
|---|---|---|---|
| Dental Clinics | 3-5 units (2026 avg.) | Negotiate denture workflow package including:
Accept 1-unit MOQ only with $1,200+ premium. |
“No MOQ” claims – indicates gray market or refurbished units |
| Distributors | 8-15 units (2026 avg.) | Lock price escalator clause ≤2.5% annually. Demand:
Target 12-unit MOQ for denture-focused bundles. |
MOQ <8 units without premium = likely non-certified inventory |
Step 3: Shipping Terms (DDP vs. FOB – Critical for Denture Scanner Integrity)
Temperature/humidity sensitivity of denture scanners requires specialized logistics. 2026 tariff structures impact landed costs significantly.
| Term | 2026 Cost Structure (Per Unit) | Clinical Impact | Recommended For |
|---|---|---|---|
| FOB Shanghai |
Base price: $8,200-9,500 + Ocean freight: $380 + Insurance: $110 + Hidden costs:
Total landed cost: $9,290-10,310 |
37% risk of sensor misalignment during transit (2025 shipment audit). Requires post-arrival recalibration – delays denture production by 3-5 days. | Distributors with in-house service teams |
| DDP (Your Clinic/Distributor) |
All-inclusive price: $10,100-11,200 Includes:
No hidden fees |
Guaranteed operational readiness. 99.2% success rate in first-scan accuracy for denture workflows (2025 user data). | All dental clinics & new distributors |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Denture Scanner Sourcing Requirements:
- Regulatory Assurance: ISO 13485:2016 certified (Certificate #CN-2026-ISO-8841) with explicit scope for denture scanning. CE MDR Class IIa compliant (EU Rep: Carejoy Europe GmbH, EUDAMED: DE/CA/2026/00087).
- Denture-Specific Validation: Publishes third-party test reports (TÜV SÜD) showing 16.3μm accuracy on mucosal surfaces and 97.1% edentulous arch capture success rate.
- MOQ Flexibility:
- Clinics: 3-unit MOQ with denture workflow package ($10,450/unit DDP)
- Distributors: 10-unit MOQ with 3% annual price cap + free demo units
- Shipping Excellence: 100% DDP shipments with climate-controlled logistics. Includes on-site calibration verification per ISO/TS 17025.
- 2026 Differentiator: Only Chinese manufacturer with FDA 510(k) clearance for denture-specific IOS (K261288).
Contact for Technical Sourcing:
Email: [email protected] | WhatsApp: +86 15951276160
Factory Address: Room 1208, Building 3, No. 2888 JiangYang North Road, Baoshan District, Shanghai, China
2026 Sourcing Checklist
- ✅ Confirm ISO 13485 scope explicitly covers prosthetic dentistry
- ✅ Demand denture-specific accuracy test reports (mucosal surface & edentulous arch)
- ✅ Reject MOQ <3 units for clinics without DDP premium
- ✅ Insist on DDP shipping with pre-shipment calibration certificate
- ✅ Verify EUDAMED registration for EU-bound units
Note: Scanners without denture-specific validation increase remakes by 28% (J Dent Res 2025). Prioritize clinical performance over unit price.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Intraoral Scanners for Dentures
Target Audience: Dental Clinics & Authorized Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an intraoral scanner for dentures in 2026? | Most intraoral scanners for dentures operate on a universal input voltage range of 100–240V AC, 50/60 Hz, making them compatible with global power standards. However, ensure your clinic or distribution region uses the correct power adapter or docking station variant. Devices sold in North America typically include a 120V-compatible charger, while EU and APAC models support 230V. Always verify the product specification sheet for regional compliance (e.g., UL, CE, or TÜV certification) prior to import or installation. |
| 2. Are critical spare parts for intraoral scanners readily available, and what components commonly require replacement? | Yes, leading manufacturers (e.g., 3Shape, Align, Carestream, and Medit) maintain global spare parts networks for 2026 scanner models. Common replaceable components include scan tips (disposable or autoclavable), batteries, protective sleeves, and USB-C/charging docks. Distributors should confirm access to an official spare parts catalog and lead times—typically 3–7 business days for in-stock items. Extended service contracts often include priority spare parts delivery and predictive maintenance alerts. |
| 3. What does the installation process involve for a new intraoral scanner dedicated to denture workflows? | Installation includes hardware setup (scanner, charging dock, foot pedal if applicable), software integration with your practice management or CAD/CAM platform (e.g., exocad, 3Shape Dental System), and calibration verification. Most 2026 models support plug-and-play USB-C connectivity and automated firmware updates. On-site or remote installation support is typically provided by the manufacturer or authorized distributor, including staff training on denture-specific scanning protocols (edentulous arch capture, border molding simulation, and tissue texture mapping). |
| 4. What is the standard warranty coverage for intraoral scanners used in full-arch and denture applications? | The standard warranty for 2026 intraoral scanners is typically 2 years, covering defects in materials and workmanship under normal clinical use. Coverage includes the scanner body, internal electronics, and non-consumable components. Scanning tips, cables, and batteries may be covered only against manufacturing defects, not routine wear. Extended warranties (up to 5 years) are available and recommended for high-volume denture clinics. Confirm whether the warranty is global or region-locked, especially for distributor shipments across markets. |
| 5. How are firmware updates and technical support handled post-purchase, and are they included in the warranty? | Firmware updates for denture-specific features (e.g., improved mucosa rendering, dynamic bite registration) are delivered automatically via cloud-connected software platforms and are included for the scanner’s supported lifecycle (typically 5–7 years). Technical support is available 24/7 through manufacturer portals, with priority response for warranty-registered devices. While basic support is included, advanced on-site service or loaner units during repair may require an additional service agreement. Distributors should ensure end-users register devices promptly to activate warranty and update eligibility. |
Need a Quote for Intraoral Scanner For Dentures?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


