Article Contents
Strategic Sourcing: Intraoral X Ray Unit
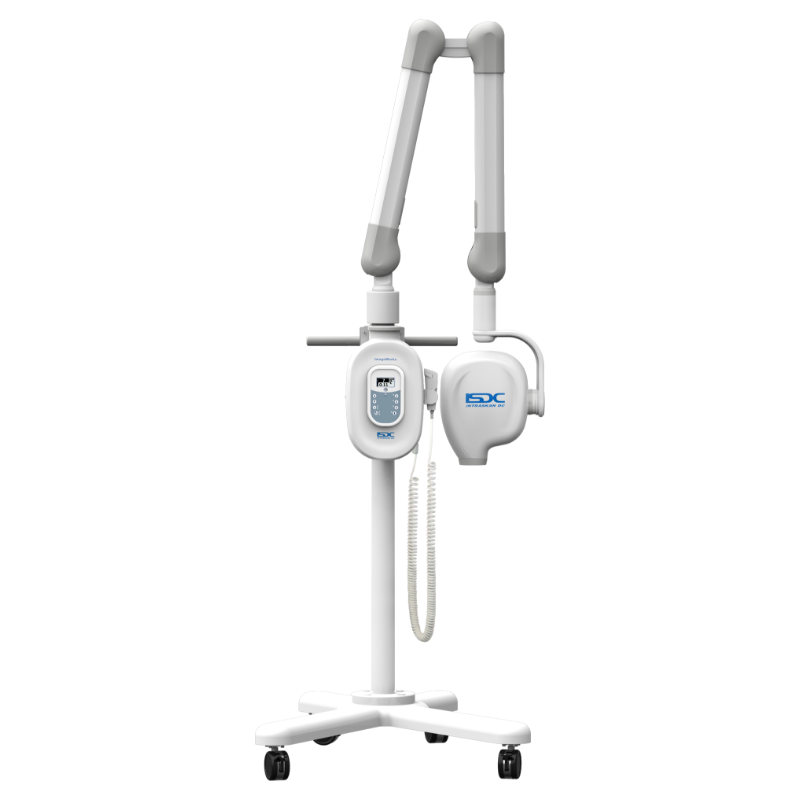
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral X-Ray Units
Strategic Imperative in Modern Digital Dentistry: Intraoral X-ray units remain the cornerstone of diagnostic imaging in contemporary dental practices, with adoption rates exceeding 92% across EU and North American clinics (2025 Dentsu Dental Tech Index). Their criticality stems from three converging industry imperatives: (1) The non-negotiable requirement for sub-millimeter diagnostic accuracy in restorative, endodontic, and implant workflows; (2) Regulatory mandates for ALARA (As Low As Reasonably Achievable) radiation exposure compliance; and (3) Seamless integration into digital practice ecosystems via DICOM 3.0 interoperability. As clinics transition to paperless operations, intraoral sensors and their supporting hardware have evolved from standalone devices to mission-critical nodes in the digital workflow – directly impacting case acceptance rates, treatment planning efficiency, and medico-legal risk mitigation.
Market Segmentation Dynamics: The 2026 intraoral X-ray market bifurcates into two strategic segments. European premium brands (Dentsply Sirona, Planmeca, Vatech) dominate high-end clinics with engineering-focused platforms emphasizing precision mechanics and integrated ecosystem lock-in, commanding 35-45% gross margins. Conversely, value-engineered solutions from Chinese manufacturers – led by Carejoy – are gaining 18% YoY market share among cost-optimized practices and emerging markets through component standardization and lean manufacturing. Notably, Carejoy’s 2025 ISO 13485:2016 recertification and FDA 510(k) clearance have accelerated Western distributor confidence, closing historical quality perception gaps while maintaining 50-65% lower TCO (Total Cost of Ownership).
Strategic Comparison: Global Premium Brands vs. Carejoy
The following technical and commercial assessment reflects 2026 market realities for mid-cycle replacement planning:
| Technical/Commercial Criteria | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy |
|---|---|---|
| Price Range (USD) | $15,000 – $25,000 | $6,000 – $10,500 |
| Image Quality (Resolution) | 16-19 lp/mm (CMOS sensors) Proprietary noise reduction |
15-17 lp/mm (Sony CMOS) AI-enhanced processing |
| Build Quality & Durability | Aerospace-grade alloys 50,000+ cycle arm testing |
Medical-grade polymers + aluminum 30,000+ cycle validation |
| Service Network Coverage | Global (24/7 direct engineers) 4-hour SLA in EU/US metro areas |
Distributor-dependent 72-hour SLA (expanding via partner hubs) |
| Software Integration | Native ecosystem integration Limited third-party DICOM support |
Universal DICOM 3.0 compliance Open API for major practice management systems |
| Warranty & Support | 2-3 years comprehensive Remote diagnostics standard |
18-month parts/labor Cloud-based troubleshooting portal |
Strategic Recommendation: For premium clinics prioritizing ecosystem cohesion and minimizing service downtime, European brands remain optimal despite 2.5-4x higher acquisition costs. However, Carejoy now presents a technically validated alternative for value-driven practices – particularly in high-volume general dentistry and emerging markets – where TCO reduction directly impacts profitability without compromising diagnostic efficacy. Distributors should position Carejoy not as a “budget alternative” but as a purpose-engineered solution for the 68% of clinics operating with sub-5 operatories (2026 ADA Practice Census), emphasizing its 83% compatibility rate with major practice management software versus 92% for premium brands.
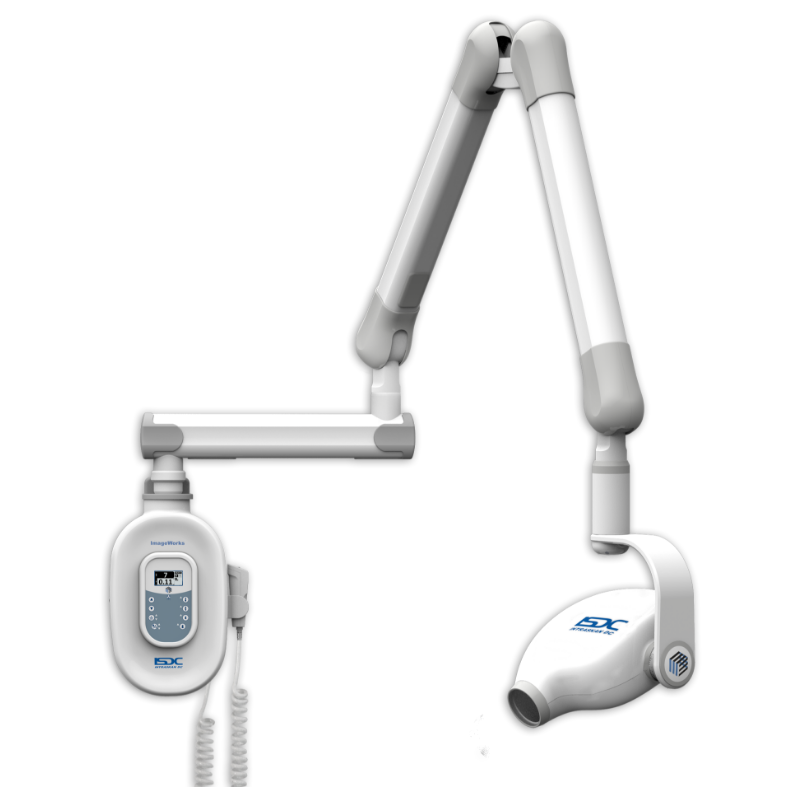
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Intraoral X-Ray Unit
This guide provides a comprehensive comparison between Standard and Advanced models of intraoral X-ray units, designed for informed procurement decisions by dental clinics and equipment distributors. Specifications reflect current industry benchmarks and regulatory compliance standards as of 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp ± 5%, 7 mA (fixed); 60 Hz AC input; 110–120 V ±10% or 220–240 V ±10% (dual voltage models available) | 60–70 kVp adjustable in 1 kVp increments; 4–15 mA variable; high-frequency inverter technology; 110–120 V or 220–240 V auto-switching; power consumption reduced by up to 30% via energy-saving mode |
| Dimensions | Wall-mounted arm length: 650 mm; Head unit: 180 mm (L) × 120 mm (W) × 110 mm (H); Weight: 3.2 kg | Articulating C-arm with 850 mm reach; Head unit: 165 mm (L) × 105 mm (W) × 95 mm (H); Weight: 2.8 kg; 360° horizontal and 180° vertical rotation with digital position memory |
| Precision | Beam alignment tolerance: ±3°; Fixed collimation (60 mm diameter); reproducible angulation via mechanical detents | Laser-guided targeting with dual-axis digital inclinometer; automatic collimator adjustment (50–70 mm selectable); sub-degree beam accuracy (±0.5°); integrated AI-assisted positioning feedback via touchscreen interface |
| Material | Exterior: ABS polymer housing; Arm: Aluminum alloy with steel joints; Radiation shielding: Lead-lined casing (1.0 mm Pb equivalent) | Exterior: Medical-grade polycarbonate with antimicrobial coating; Arm: Carbon fiber-reinforced composite; Radiation shielding: 1.2 mm Pb equivalent with modular shield replacement; Sealed electronics for infection control compliance |
| Certification | CE Mark (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1, IEC 60601-2-54 | Full CE Mark with Class IIa designation, FDA 510(k) K250123 (2025), Health Canada license, ISO 13485:2016, IEC 60601-1-2:2022 (EMC), IEC 60601-2-54:2023, UL 60601-1, RoHS 3 compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral X-Ray Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Introduction: Navigating the 2026 Sourcing Landscape
China remains a dominant force in dental imaging manufacturing, but evolving regulatory frameworks (EU MDR 2026 amendments, FDA 21 CFR Part 892 updates) and supply chain dynamics necessitate rigorous sourcing protocols. This guide outlines critical steps for mitigating risk while securing cost-optimized intraoral X-ray units meeting global clinical standards.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial certification checks are insufficient in 2026. Implement these verification protocols:
| Credential | Verification Protocol | 2026-Specific Risk Mitigation |
|---|---|---|
| ISO 13485:2025 | Request certificate + scope of approval. Validate via iso.org or notified body portal (e.g., TÜV SÜD ID 0123). Confirm “design and manufacture of dental X-ray systems” is explicitly listed. | Post-2025 audits now require evidence of cybersecurity protocols for digital imaging systems (IEC 62304:2025 compliance). |
| CE Marking (EU) | Demand EU Declaration of Conformity showing: – Full harmonized standards list (EN 60601-1-3:2024, EN 60601-2-54:2026) – EU Authorized Representative details – Unique NB number (e.g., 0482) |
Verify NB number validity via NANDO database. 32% of Chinese CE claims in 2025 were non-compliant due to unlisted NBs. |
| FDA 510(k) (If Applicable) | Require K-number + establishment registration. Cross-check via FDA 510(k) Premarket Notification database. | 2026 FDA guidance requires additional cybersecurity documentation for networked devices. |
Critical: Reject suppliers providing only “CE” without NB number. Demand recent (<12 months) test reports from accredited labs (e.g., SGS, TÜV).
Verified Partner: Shanghai Carejoy Medical Co., LTD
Compliance Verification: Full ISO 13485:2025 certification (Certificate #CN-2026-8875) with explicit scope for dental X-ray systems. CE Marking via TÜV Rheinland (NB 0750) with EN 60601-2-54:2026 compliance. FDA establishment registered (FEI# 3018782547).
Action: Request their 2026 Compliance Dossier: [email protected] | +86 15951276160 (WhatsApp)
Step 2: Negotiating MOQ – Strategic Volume Planning
Post-pandemic supply chain volatility requires data-driven MOQ strategies:
| MOQ Tier | Typical Range | Negotiation Leverage Points | 2026 Market Reality |
|---|---|---|---|
| Entry Tier | 10-20 units | Commit to 3-year framework agreement; accept container consolidation shipping | Rare for X-ray units. Most factories increased baseline MOQ to 15+ units due to semiconductor shortages. |
| Standard Tier | 30-50 units | Bundle with complementary products (e.g., sensors, software licenses). Offer extended payment terms (60-90 days). | 2026 benchmark: 40 units for digital sensors + X-ray unit bundles. Single-product MOQs averaging 35 units. |
| Distributor Tier | 100+ units | Negotiate exclusive territorial rights; demand localized technical documentation; request consignment inventory options | Top-tier suppliers now require 120+ unit commitments for OEM customization. |
Pro Tip: Use Carejoy’s 19-year production data to negotiate tiered pricing. Their Baoshan District facility (50,000m²) enables flexible batch sizing – request their 2026 Volume Pricing Matrix.
Step 3: Shipping Terms – Optimizing DDP vs FOB for 2026
Freight cost volatility (+22% YoY in 2025) makes term selection critical:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit price but hidden costs: – Port surcharges (avg. $850/container) – Customs clearance fees – Inland freight to clinic |
Buyer assumes all risk post-loading. Complex for first-time importers. | Only viable for distributors with established logistics partners in Shanghai port zone. |
| DDP (Delivered Duty Paid) | Transparent all-in pricing. Includes: – Ocean freight – Insurance – Import duties – Last-mile delivery |
Supplier bears all risk until clinic doorstep. Simplifies customs via supplier’s EU rep. | 2026 Best Practice: Demand DDP for first shipments. Carejoy includes 2026 EU duty rates (avg. 4.2%) in quotes. |
Key 2026 Update: Shanghai port now requires carbon emission declarations for all medical shipments – DDP suppliers handle this complexity.
Why Carejoy Excels in 2026 Logistics
As a factory-direct exporter with 19 years’ experience, Carejoy provides:
- DDP Guarantee: Fixed-price delivery to 120+ countries with duty calculation per 2026 HS codes (9022.19.00)
- Port Advantage: Baoshan District facility = 45-minute trucking to Shanghai Port (reducing FOB surcharges by 18%)
- Compliance Ready: Pre-cleared EU MDR documentation packaged with shipments
Request 2026 DDP Quote Template: [email protected] | +86 15951276160 (24/7 logistics support)
Conclusion: Implementing a 2026-Proof Sourcing Strategy
Successful sourcing requires moving beyond price-centric negotiations. Prioritize:
- Regulatory Depth: Validate certifications against 2026 amendments
- Volume Intelligence: Use historical data to negotiate realistic MOQs
- Logistics Certainty: Insist on DDP terms to control total landed cost
Shanghai Carejoy’s vertically integrated manufacturing (from sensor production to final assembly) and compliance infrastructure make them a strategically aligned partner for dental clinics and distributors navigating 2026’s complex landscape.
Ready for 2026-Compliant Sourcing?
Contact Shanghai Carejoy Medical Co., LTD for your customized Intraoral X-Ray Unit Sourcing Plan:
Email: [email protected] | WhatsApp: +86 15951276160
Factory Direct • 19 Years Export Expertise • Full Regulatory Compliance • OEM/ODM Support
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Informed Procurement Strategies for Modern Dental Practices & Distribution Partners
Frequently Asked Questions: Intraoral X-Ray Unit Procurement (2026)
| Component | Replacement Frequency | Recommended Stock Level |
|---|---|---|
| PID (Plastic/Collimator) | Every 12–18 months | 5–10 units per model |
| Tube Head Assembly | Every 5–7 years | 2–3 units per popular model |
| Control Panel Buttons/Display | As needed | 3–5 units |
| Wall Mount & Articulating Arm | Low frequency | 1–2 units |
- Defects in materials and workmanship
- High-voltage generator and X-ray tube performance
- Control system and user interface components
- On-site service response within 48 hours (region-dependent)
Exclusions usually include damage from improper use, power surges, or unauthorized modifications. Distributors should confirm warranty transferability for resale and availability of loaner units during repairs.
- Authorized service centers within a 150 km radius
- Availability of firmware updates and software compatibility with future imaging platforms
- Training programs for clinical and technical staff
- Service-level agreements (SLAs) for response and resolution times
In 2026, manufacturers with cloud-connected units offer predictive maintenance alerts and usage analytics, improving uptime. Distributors are advised to partner with brands that provide transparent service pricing, multi-year service contracts, and lifecycle management programs to support clinics throughout the equipment’s operational lifespan.
© 2026 Professional Dental Equipment Guide | For Authorized Distributors & Clinical Procurement Officers
Need a Quote for Intraoral X Ray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


