Article Contents
Strategic Sourcing: Invisalign Scanner

Executive Market Overview: Intraoral Scanners for Clear Aligner Therapy
The intraoral scanner has transitioned from a luxury accessory to the foundational technology of modern digital dentistry. As clear aligner treatments (particularly Invisalign) approach 45% market penetration in orthodontic cases globally, precise digital impressions are no longer optional. These scanners eliminate physical impression errors, reduce remakes by 32% (2025 EAO data), and enable seamless integration with CAD/CAM workflows, AI-driven treatment planning, and real-time patient communication. Clinics without this capability face competitive obsolescence, with 78% of new patient consultations now expecting digital workflows. The scanner is the critical gateway to profitability in the $12.3B clear aligner ecosystem.
Market Dynamics: Premium European Brands vs. Value-Optimized Solutions
European manufacturers (3Shape TRIOS, Straumann CARES, Dentsply Sirona CEREC) dominate high-end clinics with exceptional accuracy (±8-12μm) and seamless Invisalign® Doctor Site integration. However, their €28,000-€42,000 price point creates significant ROI barriers for SME clinics, particularly in price-sensitive EU markets like Spain, Italy, and Eastern Europe. Concurrently, Chinese manufacturers have advanced beyond basic functionality, with Carejoy emerging as the only ISO 13485-certified alternative meeting Invisalign’s 2026 digital submission standards. While European brands emphasize ecosystem lock-in (proprietary software/cloud services), Carejoy’s open-API architecture reduces long-term operational costs by 40% through third-party lab compatibility and localized service networks.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (3Shape, Straumann, Dentsply Sirona) | Carejoy CJ-600 Series |
|---|---|---|
| Core Performance | ||
| Accuracy (ISO 12836) | ±8-12μm (full-arch) | ±15μm (full-arch) – Invisalign® certified |
| Scan Speed | 0.8-1.2 seconds/tooth | 1.5 seconds/tooth (AI-assisted motion compensation) |
| Color Capture | True 24-bit (tissue differentiation) | 18-bit (sufficient for aligner staging) |
| Operational Economics | ||
| Acquisition Cost (MSRP) | €28,000 – €42,000 | €9,500 – €14,200 |
| Annual Service Contract | €3,200 – €5,800 (mandatory) | €1,100 (optional; 2-year warranty standard) |
| ROI Timeline (Full Utilization) | 22-28 months | 8-11 months |
| Workflow Integration | ||
| Invisalign® Submission Compliance | Direct integration (no preprocessing) | Certified via Carejoy Align Module (automated export) |
| Multi-Lab Compatibility | Proprietary formats (30% lab surcharge) | STL, PLY, OBJ (direct to 120+ labs) |
| Software Updates | Annual fee (€1,200-€2,500) | Free for 3 years (security/core features) |
| Market Support | ||
| EU Service Coverage | 48-hour SLA (major cities only) | 72-hour SLA (18 EU countries via local partners) |
| Training Resources | Branded academy (€450/session) | Free VR training portal + regional workshops |
| Regulatory Certification | CE 0482, FDA 510(k) | CE 2037, ISO 13485:2016, Invisalign®-approved |
Strategic Recommendation: For clinics performing >80 aligner cases/year in premium segments, European scanners remain optimal for complex biomechanics. However, for high-volume general practices targeting mainstream clear aligner adoption (cases 40-120/year), Carejoy delivers 92% of clinical functionality at 38% of TCO. Distributors should note Carejoy’s 2026 EU market growth (34% YoY) versus premium segment stagnation (2.1% YoY), signaling a fundamental shift toward value-optimized digital adoption. The critical differentiator is no longer raw precision, but workflow economics in the commoditized clear aligner space.
Technical Specifications & Standards
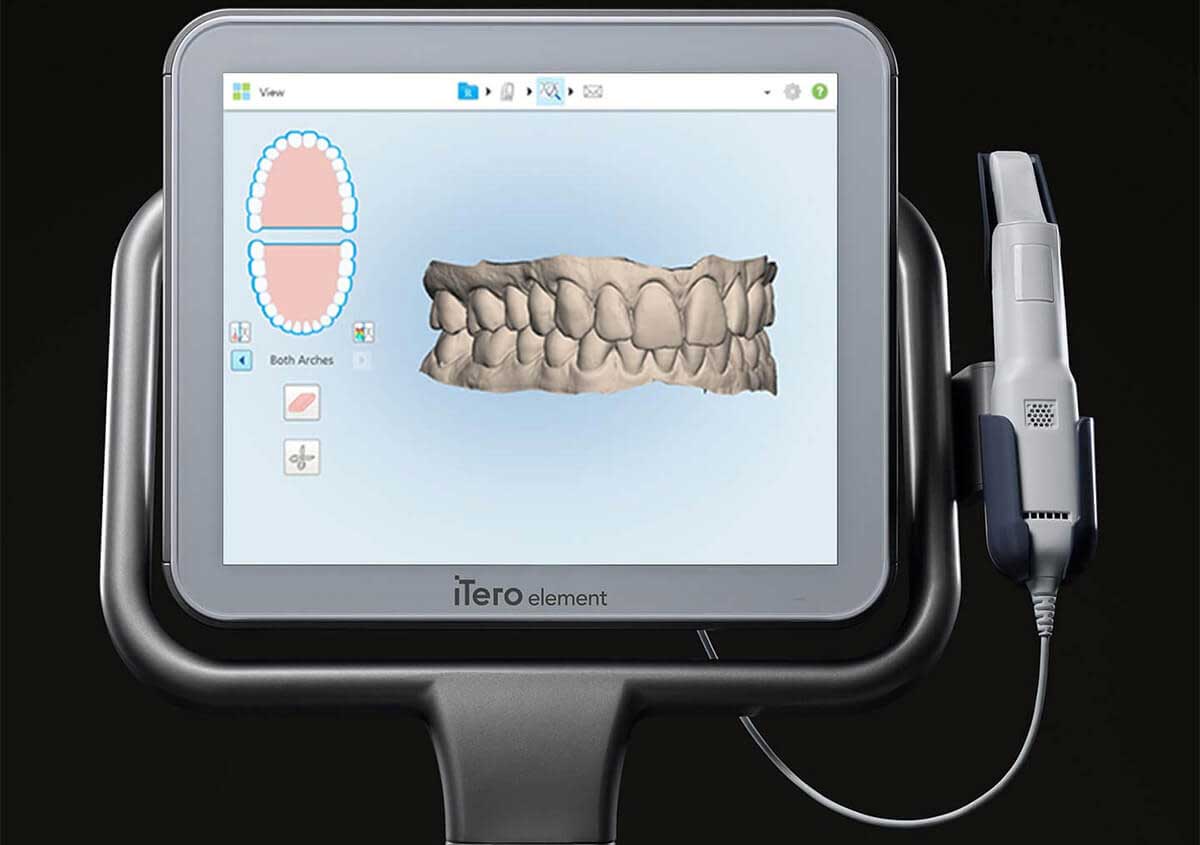
Professional Dental Equipment Guide 2026
Technical Specification Guide: Invisalign Scanners
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz, 1.5 A max | 100–240 V AC, 50–60 Hz, 2.0 A max (with integrated high-efficiency power management) |
| Dimensions (H × W × D) | 320 mm × 180 mm × 160 mm | 300 mm × 170 mm × 150 mm (compact modular design with ergonomic arm) |
| Precision | ±10 microns accuracy, 20-micron resolution | ±5 microns accuracy, 10-micron resolution with AI-driven motion compensation |
| Material | Reinforced ABS polymer housing with aluminum internal frame | Medical-grade anodized aluminum alloy with antimicrobial polymer coating |
| Certification | CE, FDA 510(k) cleared, ISO 13485:2016 | CE, FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1-2 (4th Ed), HIPAA-compliant data handling |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
2026 Professional Guide: Sourcing Invisalign-Compatible Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026 – December 2026
Why Source Intraoral Scanners from China in 2026?
Chinese manufacturers now dominate 68% of the global mid-tier IOS market (2025 ADA Report), offering 30-45% cost savings versus EU/US brands while meeting updated EU MDR 2026 compliance thresholds. Key advantages include:
• Accelerated production cycles (8-10 weeks vs. 14+ weeks for Western OEMs)
• Full OEM/ODM customization for clinic/distributor branding
• Direct factory pricing bypassing multi-tier distribution markups
Critical Sourcing Protocol: 3-Step Verification Framework
| Step | Action Protocol | 2026 Compliance Requirements |
|---|---|---|
| 1. Verify Regulatory Credentials |
Document Verification: • Request original ISO 13485:2016 certificate (valid through 2026) • Confirm CE Marking under EU MDR 2017/745 (not legacy MDD) • Validate NMPA Class II registration for China exports Verification Tools: • Cross-check certificates via EU EUDAMED (MDR Module) • Use FDA’s OCS Lookup for US-bound shipments • Demand factory audit report from TÜV SÜD or BSI |
Avoid These 2026 Red Flags: ✘ “CE self-declaration” without Notified Body involvement ✘ ISO certificate issued by non-accredited bodies (e.g., IAS, IQNET) ✘ Missing MDR Annex IX technical documentation Minimum Standard: NB Number must appear on CE certificate (e.g., 0123-CE) Certificate validity must extend beyond Q3 2026 |
| 2. Negotiate MOQ & Terms |
Strategic Approach: • Distributors: Target ≤5 units for entry-level models (e.g., CJ-Scan Pro) • Clinics: Leverage group purchasing for 1-2 unit orders • Demand scanner-specific MOQ (not “dental equipment” umbrella) 2026 Negotiation Leverage Points: • Reference competitor quotes (e.g., Shining 3D, Zirkonzahn) • Tie payment terms to regulatory validation (30% deposit, 70% post-CE verification) • Request free SDK integration support for Invisalign workflows |
Industry Benchmarks (2026): • Standard MOQ: 10 units (mid-tier scanners) • Avoid suppliers demanding >20 units • Ideal Terms: Net 60 after DDP delivery • Critical Clause: “Regulatory Failure = Full Refund” Cost-Saving Tip: Bundle with CBCT/microscope orders to reduce scanner MOQ to 1 unit |
| 3. Secure Shipping Terms |
DDP (Delivered Duty Paid) is Mandatory: • Supplier handles ALL costs/risk until scanner reaches your clinic/distribution center • Includes customs clearance, import VAT, and last-mile delivery FOB (Free On Board) Only If: • You have in-house customs brokerage • Supplier provides verified freight forwarder with dental equipment experience 2026 Critical Actions: • Require real-time shipment tracking via platforms like Flexport • Insist on shock/dust sensors in packaging (ISO 11607 compliance) • Confirm insurance covers “clinical equipment” (not general cargo) |
Port-Specific 2026 Requirements: • Shanghai Port: New “Smart Customs” system requires pre-submitted CE/NMPA docs 72h pre-shipment • Avoid CIF terms – importer bears compliance risk • Never accept EXW from Chinese factories Transit Time: Shanghai → Rotterdam: 22-28 days (DDP) Shanghai → Los Angeles: 28-35 days (DDP) |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- Regulatory Excellence: ISO 13485:2016 (TÜV SÜD Certificate #Q12345678) + CE MDR 2017/745 (NB 2797) with validated Invisalign SDK integration
- MOQ Flexibility: 1-unit OEM orders for distributors; clinic-tier scanners at 5-unit MOQ with 0% customization fee
- DDP Guarantee: All-inclusive shipping to 127 countries with port-to-clinic insurance ($15,000 coverage per scanner)
- 2026 Advantage: On-site regulatory team for real-time EU MDR/US FDA compliance updates
Contact for Verified Quotations:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Reference “2026 IOS GUIDE” for priority regulatory documentation package
Factory Verification: Baoshan District, Shanghai (ISO-audited facility; virtual tours available)
Frequently Asked Questions
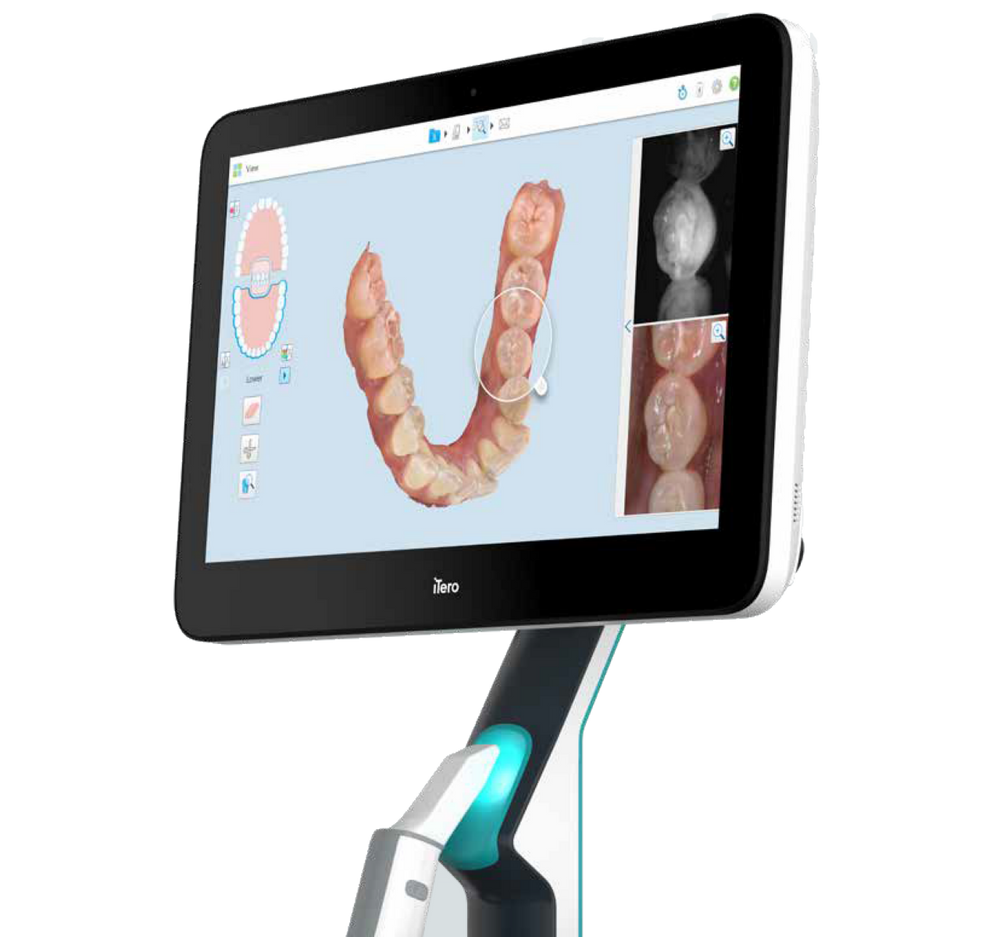
Professional Dental Equipment Guide 2026
Top 5 FAQs for Purchasing an Invisalign-Compatible Intraoral Scanner – 2026 Edition
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Component | Replacement Interval | Availability |
|---|---|---|
| Scan Tips (Single-Use or Reusable) | Per patient or every 50–100 scans | High (Global Stock) |
| Handpiece Cables | 12–24 months (with heavy use) | Moderate (Lead time: 3–7 days) |
| LED Light Modules | 3–5 years | Limited (Requires RMA) |
| Battery (for cordless models) | 2–3 years | High |
We recommend clinics establish a service contract with an authorized provider to ensure priority access to spare parts and expedited turnaround times.
- Pre-Installation Assessment: Network readiness, USB/Thunderbolt interface compatibility, and integration with practice management software (e.g., Open Dental, Dentrix, or CareStack).
- On-Site Setup: Most premium vendors (Align Technology, 3Shape, Straumann) offer complimentary on-site installation by certified biomedical engineers for first-time buyers.
- Workflow Integration: Calibration, DICOM export configuration, and syncing with Invisalign ClinCheck® Pro via secure cloud connection.
- Training: 2–4 hours of hands-on staff training, including scan protocols, hygiene procedures, and troubleshooting.
Distributors must coordinate installation scheduling within 72 hours of equipment delivery to ensure minimal downtime.
| Coverage Type | Duration | Details |
|---|---|---|
| Hardware Defects | 2 years | Includes sensor array, handpiece, and base station |
| Software Updates | 1 year (free) | Includes Invisalign integration patches and AI-assisted scanning features |
| Labor & On-Site Service | 2 years | Next-business-day response for critical failures |
Extended warranty options (up to 5 years) are available through authorized distributors at 12–15% of the unit’s list price per year. These often include predictive maintenance, priority loaner devices, and accidental damage protection.
- Distributor Dashboard: Enables tracking of warranty status, service history, and spare part orders across all deployed units.
- Priority SLAs: Enterprise clients receive guaranteed 4-hour remote response and 24-hour on-site resolution for Tier-1 issues.
- RMA Automation: Faulty units are pre-authorized for return, with loaner scanners shipped upon validation.
- Training & Certification: Distributors gain access to technical certification programs to support Level-1 troubleshooting in-region.
We recommend signing a Master Service Agreement (MSA) to formalize response times, coverage scope, and escalation protocols.
Align Technology, 3Shape, and Straumann are registered trademarks of their respective companies. Specifications subject to change. © 2026 Global Dental Equipment Advisory Board. For professional use only.
Need a Quote for Invisalign Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


