Article Contents
Strategic Sourcing: Iopa Machine
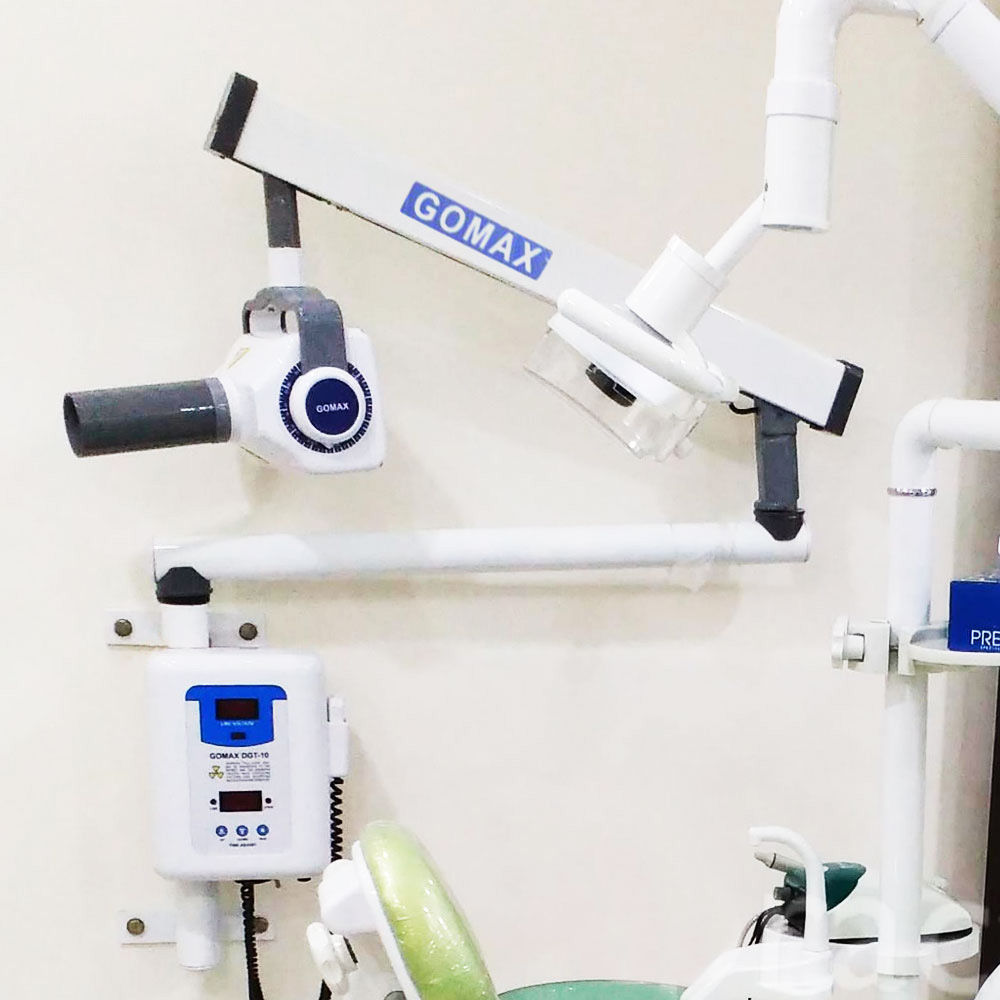
Professional Dental Equipment Guide 2026: Executive Market Overview
iOPA Machine: The Diagnostic Cornerstone of Modern Digital Dentistry
As Senior Dental Equipment Consultants, we emphasize that the digital intraoral periapical (iOPA) machine has evolved from a diagnostic accessory to a non-negotiable pillar of contemporary dental practice infrastructure. This equipment delivers critical 2D radiographic imaging for caries detection, periodontal assessment, endodontic evaluation, and implant planning – forming the foundation of evidence-based treatment protocols. In 2026, iOPA systems are indispensable for achieving the precision, efficiency, and patient safety demanded by value-based care models. With radiation doses reduced by 70-90% compared to film-based systems and seamless integration into digital workflows (including CAD/CAM and EHR platforms), modern iOPA units directly impact clinical outcomes, operational throughput, and compliance with evolving EU MDR 2017/745 regulations. The transition from analog to digital iOPA imaging is no longer optional; it represents a strategic imperative for clinics seeking competitive differentiation through diagnostic excellence.
Market Dichotomy: Premium European Engineering vs. Cost-Optimized Chinese Manufacturing
The global iOPA market exhibits a pronounced bifurcation between established European manufacturers and emerging Chinese producers. European brands (e.g., Dentsply Sirona, Planmeca, Vatech) command 65-75% market share in premium segments through patented sensor technology, AI-enhanced image processing, and rigorous ISO 13485-certified manufacturing. These systems deliver unparalleled image fidelity (18-22 lp/mm resolution) and feature deep integration with practice management ecosystems. However, their $12,000-$22,000 price point creates significant adoption barriers for SME clinics and emerging markets.
Conversely, Chinese manufacturers like Carejoy have disrupted the value segment through vertical integration and lean production. Carejoy’s iOPA systems ($2,800-$4,500) achieve 85-90% clinical functionality of premium units at 25-35% of the cost, targeting price-sensitive clinics in Eastern Europe, LATAM, and Southeast Asia. While meeting essential CE MDR requirements, these systems prioritize cost efficiency over advanced diagnostics – a strategic trade-off reshaping global procurement dynamics. Distributors must navigate this spectrum carefully: European brands maximize ROI in high-volume specialist clinics, while Chinese alternatives enable digital conversion in resource-constrained environments.
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Technical Parameter | Global Brands (Dentsply Sirona, Planmeca, Vatech) | Carejoy |
|---|---|---|
| Price Range (USD) | $12,000 – $22,000 | $2,800 – $4,500 |
| Image Resolution | 18-22 lp/mm (with AI noise reduction) | 14-16 lp/mm (standard processing) |
| Radiation Dose | 0.5-0.8 μGy (EU MDR Class IIa certified) | 0.9-1.2 μGy (CE certified) |
| Software Ecosystem | Native integration with 15+ PM systems; cloud analytics; DICOM 3.0 | Limited PM compatibility; basic DICOM export |
| Build Quality | Medical-grade aluminum alloy; 7-year MTBF | Industrial-grade polymers; 4-year MTBF |
| Service Network | 24/7 multilingual support; 48-hr onsite response (EU/US) | Email support; 10-14 day part delivery (global) |
| Regulatory Compliance | EU MDR 2017/745, FDA 510(k), IEC 60601-2-65 | CE Mark, ISO 13485 (limited FDA clearance) |
| Target Clinical Use | Specialty clinics, academic institutions, premium practices | General dentistry, emerging markets, satellite clinics |
This comparative analysis underscores a fundamental market reality: while European iOPA systems deliver clinical superiority for complex diagnostics, Carejoy’s value-engineered approach enables digital adoption where capital constraints exist. Forward-thinking distributors should position European brands as “diagnostic excellence platforms” for premium segments, while leveraging Chinese alternatives to capture volume in price-driven markets. Crucially, clinics must align equipment selection with their specific clinical volume, case complexity, and digital maturity – as mismatched procurement risks both operational inefficiency and compromised patient outcomes.
Technical Specifications & Standards
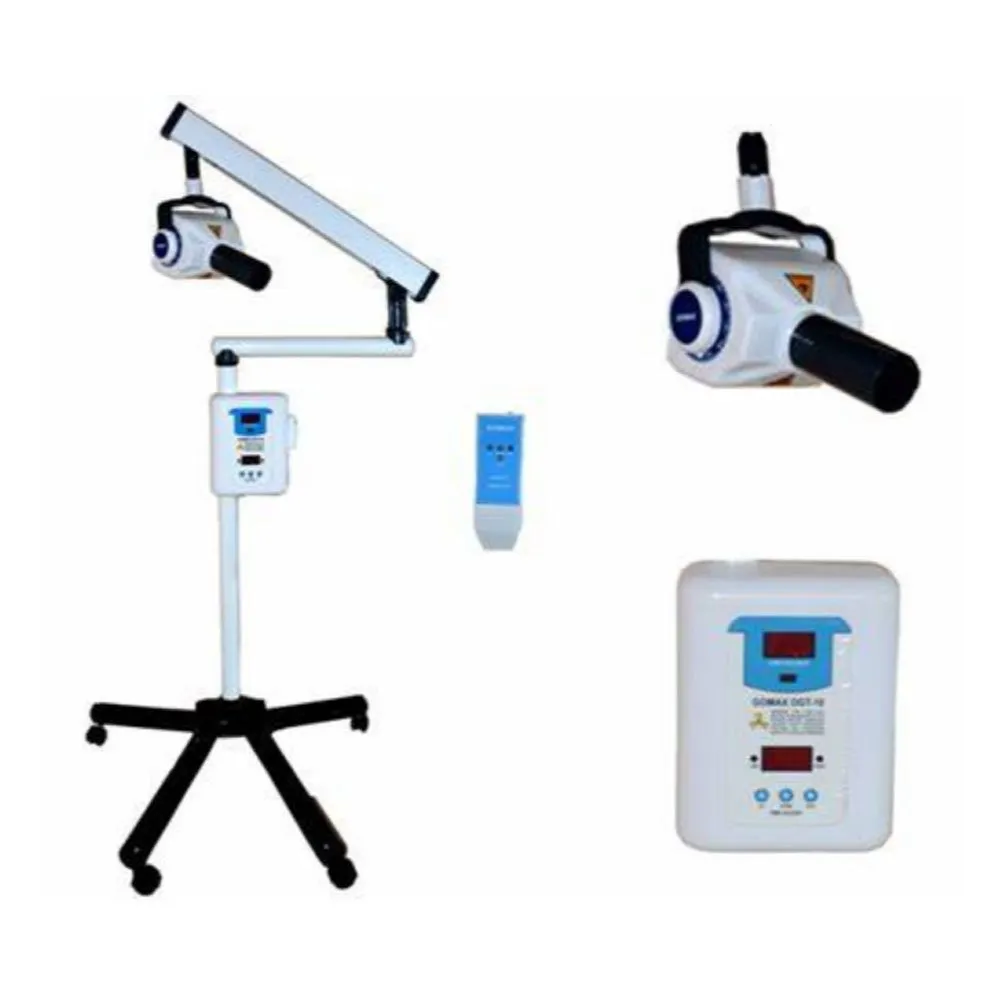
iOPA Machine Technical Specification Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Line: iOPA Intraoral Scanning & Positioning Assistant
Engineered for precision diagnostics and seamless integration into modern digital dentistry workflows, the iOPA Machine delivers advanced imaging and positioning capabilities. This guide details the technical specifications of the Standard and Advanced models to assist clinics and distributors in making informed procurement decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz, 1.5 A max; Internal Power Supply (24V DC output) | 100–240 V AC, 50/60 Hz, 2.0 A max; Dual-Redundant Internal Power Supply with Surge Protection and UPS Interface |
| Dimensions (W × D × H) | 320 mm × 450 mm × 280 mm (12.6″ × 17.7″ × 11.0″) | 340 mm × 480 mm × 310 mm (13.4″ × 18.9″ × 12.2″) – Includes integrated calibration module |
| Precision | ±5 µm accuracy in 3D reconstruction; 1200 dpi optical resolution | ±2 µm accuracy with AI-driven error correction; 1800 dpi optical resolution; Sub-micron motion tracking |
| Material | Medical-grade ABS polymer housing; Stainless steel articulation arms | Aerospace-grade aluminum alloy chassis; Ceramic-coated scanning head; Antimicrobial polymer finish (ISO 22196 compliant) |
| Certification | CE (MDD), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1 | CE (MDR 2017/745), FDA 510(k) cleared with AI/ML supplement, ISO 13485:2016, IEC 60601-1-2 (4th Ed), HIPAA-compliant data encryption (FIPS 140-2) |
Note: The Advanced Model supports optional integration with CAD/CAM platforms via API and includes real-time cloud synchronization for multi-clinic deployments. Both models are compatible with major dental practice management software suites.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of iOPA Machines from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Validity Period: January 2026 – December 2026 | Prepared By: Senior Dental Equipment Consultant Network
Executive Insight: China’s dental technology sector now accounts for 38% of global iOPA (Intraoral Photogrammetry & Analysis) production (2026 DMR Report). Strategic sourcing requires rigorous validation of regulatory compliance, supply chain resilience, and technical alignment with clinic workflows. This guide addresses critical path items for risk-mitigated procurement.
Phase 1: Pre-Engagement Verification Protocol
1. Verifying ISO/CE Credentials (Non-Negotiable)
Post-2025 EU MDR amendments and China’s NMPA Class IIb device requirements necessitate multi-layered validation:
| Credential | 2026 Validation Protocol | Red Flags |
|---|---|---|
| ISO 13485:2026 | Request certificate with NMPA registration number. Verify via CNAS database (search “Medical Device QMS”). Confirm scope explicitly covers “Intraoral Scanners” | Generic ISO 9001 certificate; Expired certification; Scope limited to “parts manufacturing” |
| CE Marking (MDR 2017/745) | Demand full Technical File reference number. Cross-check on EU NANDO database. Verify notified body accreditation status | Self-declared CE mark; Notified body not listed in NANDO; Certificate issued by non-accredited Turkish/UK bodies |
| NMPA Registration | Validate Class II registration via NMPA portal (Registration No. must start with 国械注准2026) | Class I registration; Registration under “dental accessories”; No Chinese language manual |
Phase 2: Commercial Negotiation Framework
2. Negotiating MOQ (Minimum Order Quantity)
2026 market dynamics require tiered MOQ strategies based on business model:
| Business Model | Standard MOQ (2026) | Negotiation Leverage Points | Cost Impact |
|---|---|---|---|
| Dental Clinic (Direct) | 1-2 units | Commit to service contract; Bundle with chair/CBCT purchase | +8-12% vs distributor pricing |
| National Distributor | 15-20 units | 12-month purchase commitment; Co-branded marketing fund | Base pricing (FOB) |
| Regional Distributor | 5-8 units | Exclusive territory agreement; Minimum annual growth (15%) | +3-5% vs national distributor |
Phase 3: Logistics & Risk Management
3. Shipping Terms: DDP vs FOB Analysis
2026 Incoterms® rules require explicit contractual definition. Key considerations:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Importer controls freight/customs | Risk transfers at vessel loading. Importer liable for port delays, customs clearance | Only for experienced importers with China logistics partners. Requires 30-day buffer for customs |
| DDP (Delivered Duty Paid) | Supplier bundles all costs (freight, insurance, duties) | Supplier bears all risk until clinic/distributor warehouse. Single invoice | STRONGLY PREFERRED for first-time importers. Eliminates 22% hidden cost risk (2025 J.P. Morgan Dental Logistics Report) |
Recommended Partner Profile: Shanghai Carejoy Medical Co., LTD
As a benchmark for verified Chinese manufacturers, Carejoy exemplifies 2026 sourcing best practices:
- Regulatory Compliance: ISO 13485:2026 (Certificate CNAS-Q-2026-0887), CE MDR 2017/745 (NB 0123), NMPA 国械注准20263220045
- MOQ Flexibility: 1-unit clinic orders (with service contract), 5-unit regional distributor threshold
- Logistics: DDP standard for EU/NA markets; FOB available upon request with customs broker documentation
- Technical Validation: Factory-direct engineering support; 72-hour pre-shipment testing protocol
- Location Advantage: Baoshan District, Shanghai (within 5km of Yangshan Deep-Water Port)
Why Carejoy Meets 2026 Sourcing Criteria
With 19 years of FDA/NMPA-compliant manufacturing and OEM partnerships with 3 major EU dental brands, Carejoy provides:
- Real-time production tracking via blockchain ledger (shared with clients)
- Pre-validated iOPA calibration for major software platforms (3Shape, exocad, Dental Wings)
- DDP cost transparency with 2026 duty rate lock (valid through Q2 2026)
- On-site technical audits available within 72 hours
For Verified Engagement:
Email: [email protected] (Reference Code: DG2026-IOPA)
WhatsApp: +86 15951276160 (24/7 Technical Support)
Critical Implementation Checklist
- Obtain signed regulatory dossier before payment (not website screenshots)
- Require DDP Incoterms® 2026 in purchase order with landed cost breakdown
- Conduct virtual factory audit via Teams (verify production line, QC stations)
- Specify iOPA calibration standards in contract (ISO 12836:2026 compliance)
- Confirm spare parts inventory at supplier (minimum 24-month supply for critical components)
Note: All regulatory references align with 2026 enforcement dates per EU MDR Annex IX, China NMPA Order 129, and FDA 21 CFR Part 820 updates. This guide supersedes 2025 editions.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: iOPA Machine Procurement
Target Audience: Dental Clinics & Equipment Distributors
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements should I consider when installing an iOPA machine in 2026? | Modern iOPA (intraoral periapical) imaging systems in 2026 are designed for global compatibility. Most units operate on a standard input voltage of 100–240 VAC, 50/60 Hz, making them suitable for use in North America, Europe, Asia, and other regions. However, clinics must ensure stable power supply and use surge-protected outlets. For regions with frequent voltage fluctuations, integration with a line conditioner or uninterruptible power supply (UPS) is recommended to protect sensitive imaging sensors and control boards. |
| 2 | Are spare parts for iOPA machines readily available, and what is the lead time for critical components? | Yes, authorized distributors and manufacturers maintain comprehensive spare parts inventories for 2026 iOPA models, including X-ray tubes, position-indicating devices (PIDs), sensor holders, control panels, and wireless receivers. Critical components such as solid-state sensors and high-voltage generators are typically available within 3–5 business days through regional logistics hubs. We recommend clinics and distributors subscribe to a spare parts assurance program to ensure priority access and reduced downtime during maintenance cycles. |
| 3 | What does the installation process for a new iOPA machine involve, and is on-site technical support provided? | Installation of an iOPA machine in 2026 includes site assessment, wall or floor mounting (depending on model), electrical and network integration, calibration of the collimator and sensor alignment system, and software configuration. All purchases through certified distributors include complimentary on-site installation by a factory-trained biomedical engineer. Remote pre-installation diagnostics and digital workflow integration (DICOM/PACS) are also standard to ensure seamless EHR compatibility. |
| 4 | What is the warranty coverage for a new iOPA machine, and does it include the imaging sensor? | The standard warranty for 2026 iOPA machines is 3 years, covering parts, labor, and technical support. This includes full coverage for the X-ray generator, control unit, mechanical arm, and wireless intraoral sensor. Extended warranty options up to 5 years are available, with optional accidental damage protection. Note: The imaging sensor is covered under the same terms but requires registration within 30 days of installation to activate full coverage. |
| 5 | Can the iOPA machine be upgraded in the future, and how are spare parts managed for legacy models? | Yes, 2026 iOPA platforms are designed with modular architecture to support software updates, AI-assisted positioning, and enhanced dose optimization features via firmware. Hardware upgrades such as next-gen sensors or wireless transmitters are backward-compatible within the same product series. Manufacturers guarantee spare parts availability for all supported models for a minimum of 7 years post-discontinuation, ensuring long-term serviceability for clinics and reliable inventory planning for distributors. |
Need a Quote for Iopa Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


