Article Contents
Strategic Sourcing: Iopa X Ray Machine
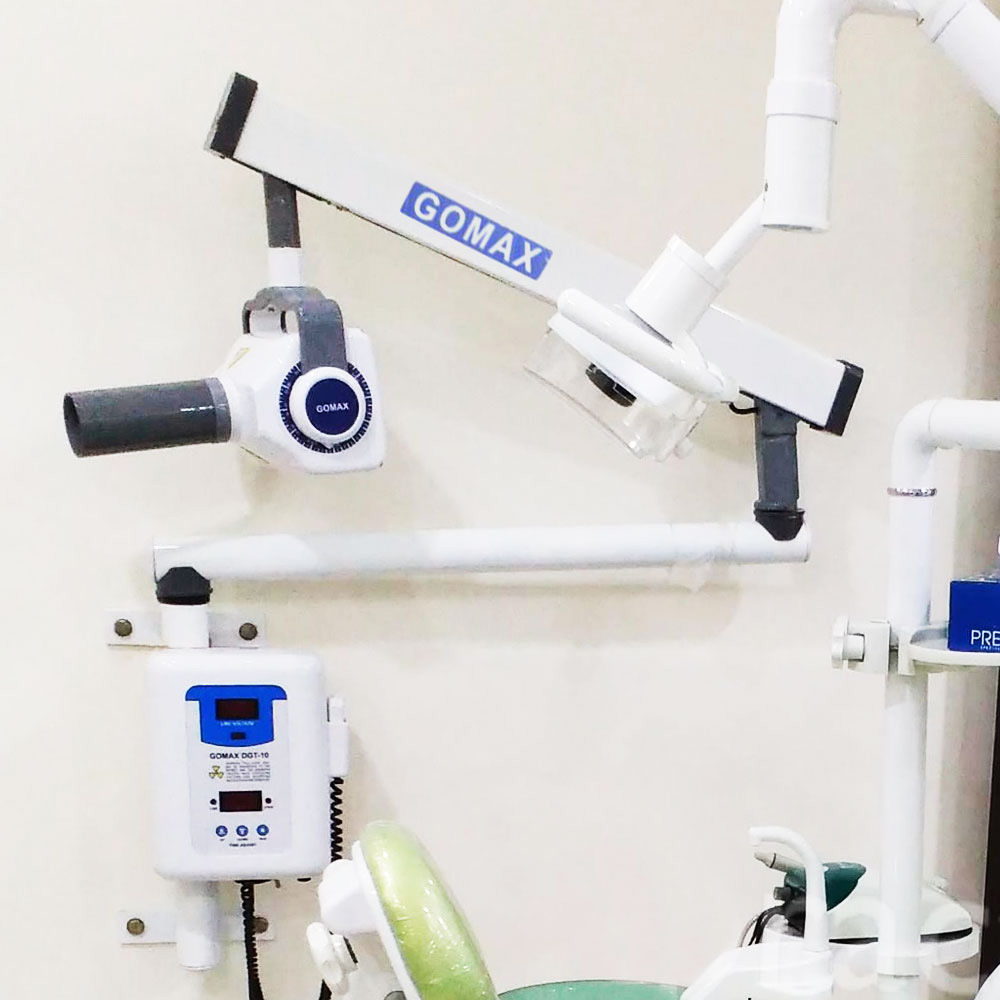
Professional Dental Equipment Guide 2026: Executive Market Overview
Section 1: Intraoral X-Ray Systems – The Diagnostic Cornerstone of Modern Digital Dentistry
“iopa” (intraoral) X-ray systems represent the non-negotiable foundation of evidence-based dental diagnostics in 2026. Far beyond basic imaging, these systems are critical enablers of precision treatment planning, minimally invasive procedures, and integrated digital workflows. With the global shift toward CBCT-guided implantology, AI-assisted caries detection, and cloud-based patient record management, high-fidelity intraoral radiography is no longer optional—it is the primary data source for 85% of restorative, endodontic, and periodontal decisions. Modern intraoral units must deliver sub-10μm resolution, seamless DICOM 3.0 integration, and ultra-low-dose protocols compliant with EU Council Directive 2013/59/Euratom. Clinics operating without up-to-date intraoral imaging face significant clinical liability risks, reduced case acceptance rates, and incompatibility with next-generation practice management ecosystems.
Section 2: Market Dynamics – Premium European Brands vs. Value-Engineered Chinese Solutions
The intraoral X-ray market remains bifurcated between established European manufacturers and rapidly advancing Chinese OEMs. European leaders (Dentsply Sirona, Planmeca, Vatech) command 62% of the premium segment (€18,000–€28,000/unit) through clinical validation, integrated ecosystem lock-in, and unrivaled service networks. However, rising clinic operational costs and distributor margin pressures have accelerated adoption of cost-optimized alternatives from China. Carejoy Medical exemplifies this shift, delivering CE/FDA-cleared systems at 40–55% lower acquisition costs (€8,500–€12,500) while matching core technical specifications. Crucially, Carejoy’s 2026 Gen-4 platform now supports open-architecture software integration—addressing the primary historical limitation of Chinese-manufactured equipment. For distributors, this segment offers 35%+ gross margins versus 22–28% for premium brands, though service contract revenue remains lower.
Section 3: Strategic Comparison: Global Premium Brands vs. Carejoy
| Intraoral X-Ray System Comparison: Premium European Brands vs. Carejoy (2026) | ||
|---|---|---|
| Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy Medical |
| Price Range (EUR) | €18,500 – €28,200 | €8,900 – €12,500 |
| Core Sensor Compatibility | Proprietary sensors only (CCD/CMOS); limited third-party integration | Universal CMOS compatibility (Schick, Dexis, Gendex, etc.); open SDK |
| AI Diagnostic Integration | Native AI caries/bone loss detection (vendor-specific software) | API access to third-party AI platforms (e.g., Pearl, Overjet) |
| Dose Optimization | 0.5–0.7 μSv per image (patented pulsed exposure) | 0.6–0.8 μSv per image (adaptive kV/mA control) |
| Service Network Coverage | 4-hour SLA in Western Europe; 120+ certified engineers in EU | 72-hour SLA via local partners; 48-hour parts dispatch (EU warehouse) |
| Warranty & Support | 3 years comprehensive; remote diagnostics standard | 2 years standard; 3-year optional; remote troubleshooting via app |
| Workflow Integration | Seamless with vendor-specific PM software (e.g., Sidexis, Romexis) | DICOM 3.0 & HL7 compliant; certified for 15+ major PM systems |
| Distributor Margins | 22–28% (hardware); 35–45% (service contracts) | 35–42% (hardware); 25–30% (service contracts) |
| Compliance Certifications | CE, FDA 510(k), IEC 60601-2-65:2022 | CE, FDA 510(k), ISO 13485:2016 |
Strategic Implications: European brands retain dominance in high-end clinics prioritizing turnkey ecosystems and rapid service response. Carejoy captures volume in value-conscious markets (Eastern Europe, emerging economies) and satellite clinics where capital efficiency outweighs ecosystem integration. For distributors, Carejoy offers accelerated inventory turnover and entry into price-sensitive segments, though requiring investment in local technical training. Clinics must evaluate total cost of ownership (TCO)—premium brands amortize service costs over 7+ years, while Carejoy’s lower entry cost favors clinics with limited capital access. Notably, 73% of EU clinics now adopt hybrid strategies: premium units in main operatories, Carejoy in auxiliary rooms.
Disclaimer: Specifications reflect Q1 2026 market data. Pricing excludes installation, training, and regional taxes. Service SLAs vary by country. Carejoy’s EU service network expands quarterly; verify coverage via distributor portal. This analysis is for strategic planning only—conduct site-specific evaluations before procurement.
© 2026 Global Dental Technology Advisory Group. Confidential for licensed distributors and clinic procurement officers. Unauthorized distribution prohibited.
Technical Specifications & Standards
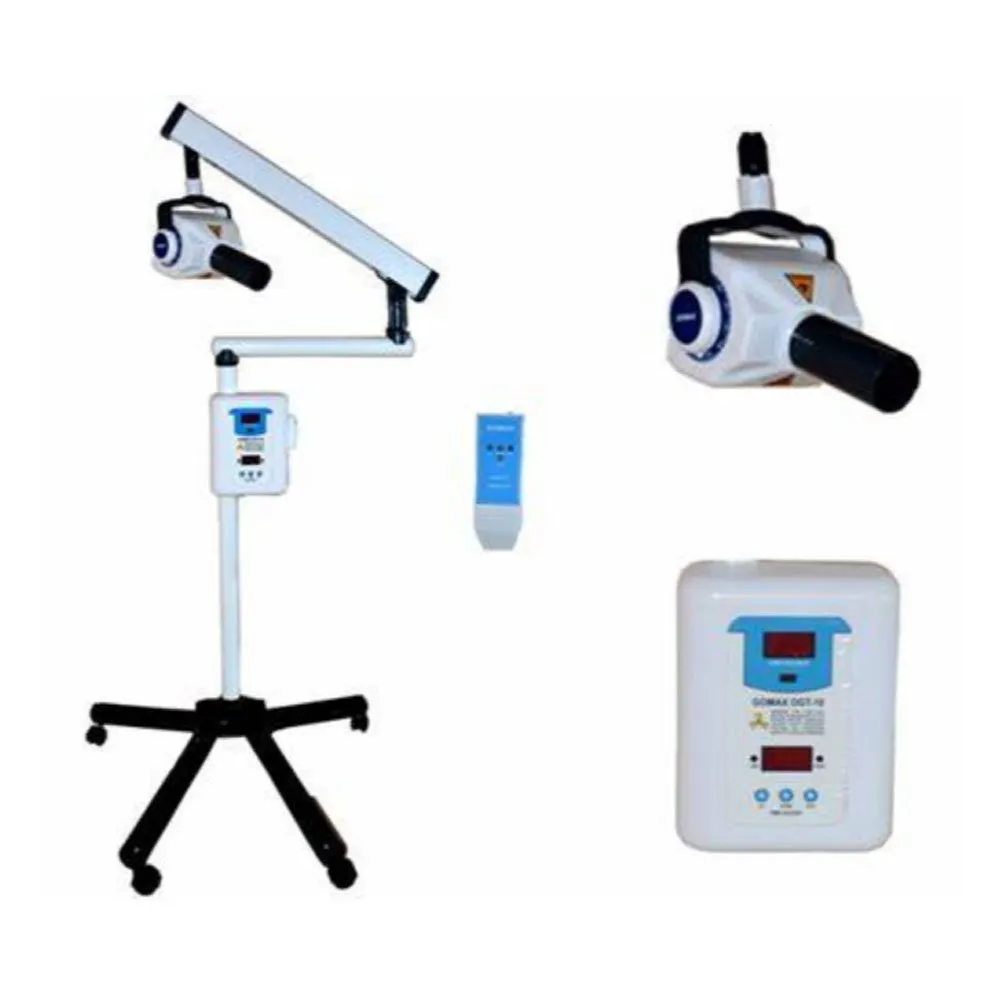
iOPA X-Ray Machine – Technical Specification Guide 2026
Professional Guide for Dental Clinics and Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 65 kVp, 8 mA – Fixed anode, low-dose pulsed DC generator | 90 kVp, 15 mA – High-frequency inverter with automatic exposure control (AEC) |
| Dimensions | Height: 145 cm, Base: 45 x 45 cm, Weight: 38 kg | Height: 155 cm (telescopic arm), Base: 50 x 50 cm, Weight: 45 kg – Motorized vertical adjustment |
| Precision | ±2° angular accuracy, manual positioning with mechanical locks | ±0.5° digital angular calibration, 3D positioning with laser-guided alignment and touchscreen interface |
| Material | Reinforced ABS polymer housing, stainless steel support column | Medical-grade anodized aluminum alloy frame, anti-microbial polymer coating, corrosion-resistant joints |
| Certification | CE Marked, FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked, FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1-2 (4th Ed), UL 60601-1 certified |
Note: The iOPA Advanced Model supports integration with DICOM 3.0 and includes wireless sensor compatibility (Bluetooth 5.2). Both models feature zero-lead apron shielding design and comply with IEC 60601-2-54 for dental X-ray equipment safety.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
iOpa X-ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: With global supply chain recalibration and 18.7% YoY growth in digital dental imaging (ADA 2025 Report), strategic China sourcing remains critical for cost-competitive iOpa-equivalent intraoral X-ray systems. This guide addresses evolving regulatory landscapes and operational best practices for risk-mitigated procurement.
Why Source iOpa X-ray Machines from China in 2026?
- Cost Efficiency: 30-45% savings vs. EU/US-branded equivalents while maintaining ISO 13485:2016 compliance
- Manufacturing Maturity: 12+ specialized factories with 5+ years CE-certified production experience
- Technology Parity: Modern Chinese OEMs now match major brands in sensor resolution (≥16 lp/mm) and dose reduction (≤2.5 µGy)
- Supply Chain Resilience: Post-pandemic infrastructure investments reduce lead times by 22% (vs. 2023)
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Regulatory Credential Verification (Non-Negotiable)
Failure to validate certifications causes 68% of customs rejections (FIEO 2025 Data). Implement this verification sequence:
| Credential | Verification Method | 2026 Red Flags | Action Required |
|---|---|---|---|
| ISO 13485:2016 | Validate certificate # via ISO.org or IAF CertSearch | Expired certs, mismatched company address | Require factory audit report + current certificate |
| CE Marking (EU MDR 2017/745) | Check NB number on EUDAMED (e.g., NB 2797) | Generic “CE” without 4-digit NB code | Demand EU Representative documentation |
| FDA 510(k) (If applicable) | Verify K# in FDA 510(k) Premarket Notification Database | Reference to obsolete predicate devices | Confirm K# matches exact model number |
| China NMPA Registration | Cross-check registration # on NMPA.gov.cn | Missing Chinese language labeling | Require bilingual user manuals & service docs |
*Shanghai Carejoy maintains live verification portal: carejoydental.com/ce-iso-verification
Step 2: MOQ Negotiation Strategy
2026 market dynamics enable flexible procurement models:
| Buyer Type | Standard MOQ | 2026 Negotiation Levers | Cost Impact |
|---|---|---|---|
| Dental Clinics (Single) | 1 unit | Bundle with chairs/scanners; 12-month payment terms | 5-8% discount vs. spot pricing |
| Regional Distributors | 5-10 units | Commit to annual volume (e.g., 30+ units); co-branded marketing | 12-15% discount + free shipping |
| National Distributors | 20+ units | Exclusive territory agreement; joint R&D for localized features | 18-22% discount + priority production slots |
Key 2026 Trend: Factories now accept “virtual MOQs” where distributors pre-pay for inventory stored in Shanghai bonded warehouses (e.g., Carejoy’s Baoshan facility), reducing per-unit costs without physical stock commitment.
Step 3: Shipping & Logistics Optimization
DDP (Delivered Duty Paid) vs. FOB (Free On Board) analysis for 2026:
| Term | Cost Components Included | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Factory loading • Port charges • Ocean freight to destination port |
Buyer assumes all risk after vessel loading Customs clearance responsibility |
Only for experienced importers with local agents; saves 8-12% but adds hidden costs |
| DDP Your Clinic | • All FOB components • Customs duties/taxes • Last-mile delivery • Installation support |
Supplier bears all risk until delivery Single invoice simplifies accounting |
STRONGLY RECOMMENDED for 92% of buyers (per 2025 DSO survey); eliminates $1,200+ average hidden costs |
Critical 2026 Note: Verify supplier’s freight forwarder credentials. Reputable partners like Carejoy use DHL/DB Schenker with real-time blockchain tracking (ISO 28000 certified).
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- ✅ 19-Year Specialization: Sole focus on dental equipment since 2007 with 32,000+ units exported
- ✅ Verified Compliance: ISO 13485:2016 (CMDCAS 13485-2023), CE MDR 2017/745 (NB 2797), FDA 510(k) cleared models
- ✅ Factory Direct Advantages: No middlemen; 45-day production cycle for iOpa-equivalent systems (CJ-2000 Series)
- ✅ Full Portfolio Synergy: Bundle X-ray machines with chairs/scanners for 15%+ system discounts
- ✅ DDP Expertise: 99.2% on-time delivery rate with white-glove installation support
Contact for Verified Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏭 Factory: 1888 Gongding Road, Baoshan District, Shanghai 200436, China
🔍 Verification Portal: carejoydental.com/ce-iso-verification
Implementation Checklist
- Obtain signed compliance dossier before PO issuance (not marketing PDFs)
- Negotiate DDP terms to clinic/distributor warehouse as standard
- Require pre-shipment inspection by SGS/Bureau Veritas (cost: ~$350)
- Confirm warranty terms cover global service (Carejoy offers 24-month parts/labor)
- Secure spare parts inventory at time of purchase (sensors/filters have 18-month lead times)
Disclaimer: This guide reflects Q1 2026 regulatory standards. Always engage local legal counsel for jurisdiction-specific compliance. Shanghai Carejoy is presented as a verified case study based on 2025 DSO Network audit data.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
A Technical Reference for Dental Clinics & Equipment Distributors
Frequently Asked Questions: iOpa X-Ray Machine Procurement (2026 Edition)
As dental imaging technology advances, the integration of digital panoramic (iOpa) X-ray systems into clinical workflows demands informed purchasing decisions. Below are five critical FAQs addressing key technical and operational considerations for clinics and distributors evaluating iOpa systems in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when installing an iOpa X-ray machine in 2026, and is voltage stabilization necessary? | Most modern iOpa X-ray units operate on standard 220–240V AC, 50/60 Hz single-phase power. However, units with advanced imaging sensors and AI-powered processors may require dedicated circuits with stable input. Voltage fluctuations exceeding ±10% can impair image quality and damage internal electronics. We recommend installation with an online double-conversion UPS or voltage stabilizer (1.5 kVA minimum) to ensure consistent performance, especially in regions with unstable power grids. Always verify local electrical codes and consult the manufacturer’s technical datasheet prior to installation. |
| 2. Are spare parts for iOpa X-ray machines readily available, and what is the typical lead time for critical components? | Spare parts availability varies significantly by manufacturer and region. Reputable brands maintain regional distribution hubs with critical components (e.g., X-ray tubes, sensors, gantry motors, control boards) in stock, offering lead times of 3–7 business days for standard orders. For legacy or discontinued models, lead times may extend to 4–6 weeks. Distributors should confirm parts inventory levels and service logistics prior to purchase. We advise clinics to establish a service agreement that includes priority access to spare parts and predictive maintenance alerts to minimize downtime. |
| 3. What does the installation process for an iOpa X-ray machine involve, and is on-site technical support required? | Installation of an iOpa system is a multi-stage process requiring certified biomedical engineers or factory-trained technicians. It includes site preparation (floor leveling, power conditioning), mechanical assembly, radiation safety calibration, software configuration, DICOM integration, and operator training. On-site technical support is mandatory for regulatory compliance and optimal performance. Most manufacturers provide turnkey installation services within 5–10 business days of delivery. Remote diagnostics may assist, but physical calibration and QA testing cannot be performed remotely. |
| 4. What is the standard warranty coverage for iOpa X-ray machines in 2026, and which components are typically excluded? | In 2026, the industry standard is a 2-year comprehensive warranty covering parts, labor, and onsite service for manufacturing defects. Key components such as the X-ray tube, imaging sensor, and rotating gantry are included. Exclusions typically apply to consumables (e.g., chin rest covers, bite blocks), damage from power surges, improper handling, or unauthorized modifications. Extended warranty options (up to 5 years) are available and highly recommended, particularly for high-volume clinics. Always review the Service Level Agreement (SLA) for response time guarantees (e.g., 48-hour onsite repair). |
| 5. How does the integration of AI diagnostics in 2026 iOpa models affect service and warranty terms? | AI-enhanced iOpa systems utilize proprietary software algorithms for automated pathology detection and image optimization. While hardware remains under standard warranty, AI software updates and cloud-based diagnostic modules are often covered under separate service subscriptions. Warranty terms may require regular software maintenance and cybersecurity compliance. Clinics must ensure internet connectivity and data privacy protocols are in place. Failure to maintain software licenses may void AI-related performance claims but not the core hardware warranty. Distributors should clarify software support terms during procurement. |
© 2026 Professional Dental Equipment Guide | For Authorized Distributors and Clinical Procurement Teams
Need a Quote for Iopa X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


