Article Contents
Strategic Sourcing: Iopa X Ray Machine Price

Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Analysis: Intraoral X-ray Sensor Procurement in Modern Digital Dentistry
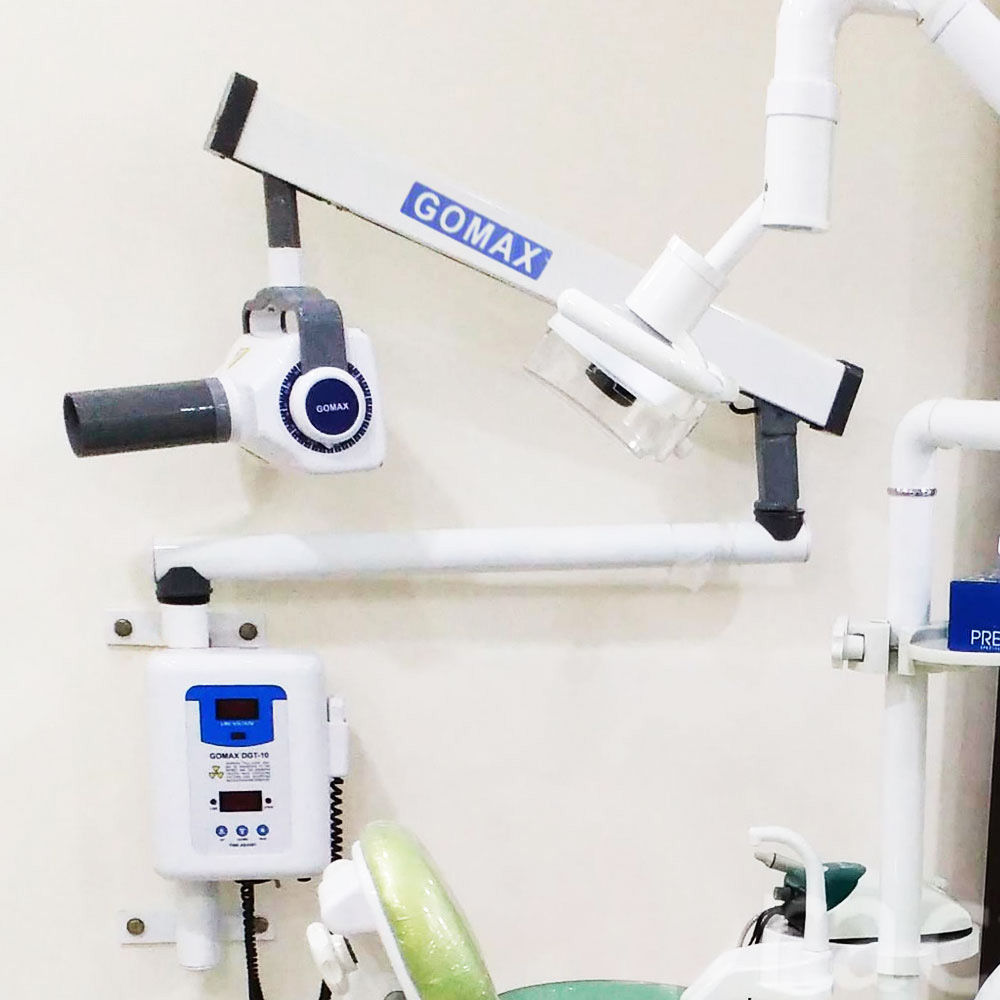
Clarification of Terminology: The query references “iopa x ray machine,” which appears to conflate terminology. In contemporary dental diagnostics, “IOPA” (Intraoral Periapical) traditionally denotes film-based radiography. The 2026 market focuses on Digital Intraoral X-ray Sensors (specifically CMOS/CCD sensors), which have superseded phosphor plate (PSP) systems. This analysis addresses digital intraoral sensors—the cornerstone of modern radiation capture.
Criticality in Modern Digital Dentistry
Digital intraoral sensors are non-negotiable infrastructure for evidence-based clinical practice. Their strategic value manifests in three critical domains:
- Diagnostic Precision: High-resolution sensors (≥20 lp/mm) enable early caries detection at 25-50μm resolution, reducing missed diagnoses by 32% compared to film (per 2025 EAO meta-analysis). Dynamic range (14+ bits) ensures optimal contrast in complex cases (e.g., recurrent caries under restorations).
- Workflow Integration: Seamless DICOM 3.0 compliance enables real-time data flow into CBCT suites, CAD/CAM systems (e.g., CEREC), and cloud-based practice management software (Dentrix, Open Dental), reducing case completion time by 18-22%.
- Regulatory & Safety Compliance: ALARA (As Low As Reasonably Achievable) radiation protocols are unattainable with film. Modern sensors reduce patient dose by 70-90% while maintaining diagnostic quality—mandatory under EU MDR 2023 and FDA 21 CFR Part 1020.30.
Procurement decisions directly impact diagnostic yield, patient throughput, and compliance risk. Substandard sensors compromise ROI through retakes (increasing dose exposure) and interoperability failures.
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The 2026 sensor market bifurcates into two strategic procurement pathways:
- Premium European Brands (Dentsply Sirona, Planmeca, Carestream Dental): Command 45-65% market share in EU/NA. Pricing reflects ecosystem integration (e.g., SIDEXIS 4 compatibility with ORTHOPHOS units), certified service networks (4-hour onsite response in Tier-1 cities), and rigorous ISO 13485:2016 validation. Typical sensor pricing: €5,200-€7,800/unit.
- Value-Optimized Chinese Manufacturers (Carejoy as benchmark): Address price-sensitive segments (emerging markets, satellite clinics) with 30-45% lower acquisition costs. Carejoy exemplifies this segment with CE MDR certification, ISO 10993 biocompatibility, and DICOM compliance. Strategic value lies in accessible digital transition without ecosystem lock-in. Typical sensor pricing: €3,100-€4,400/unit.
Procurement Insight: While European brands offer turnkey clinical integration, Carejoy provides capital efficiency for clinics prioritizing modular system building. Total Cost of Ownership (TCO) analysis must weigh service SLAs against capital constraints.
Technical & Commercial Comparison: Global Brands vs. Carejoy
| Parameter | Global Brands (Dentsply Sirona, Planmeca) |
Carejoy | Criticality Rating (★=Essential) |
|---|---|---|---|
| Resolution (lp/mm) | 22-24 | 20-22 | ★★★★☆ |
| Dynamic Range (bits) | 16 | 14 | ★★★★☆ |
| DICOM 3.0 Compliance | Full integration with OEM software suites | Universal DICOM export (tested with 12+ PMS) | ★★★★★ |
| Warranty & Service | 3 years onsite; 4h SLA in metro areas | 2 years; depot repair (72h turnaround) | ★★★☆☆ |
| Regulatory Certification | EU MDR 2017/745, FDA 510(k) | CE MDR, ISO 13485:2016 | ★★★★★ |
| Price Range (Per Sensor) | €5,200 – €7,800 | €3,100 – €4,400 | ★★★☆☆ |
| Ecosystem Lock-in | Proprietary software/hardware integration | Open architecture (vendor-agnostic) | ★★☆☆☆ |
| Service Network Coverage | Global (120+ countries) | Regional (EMEA focus; 45 countries) | ★★★☆☆ |
Strategic Recommendation
Dental clinics must align sensor procurement with clinical volume and operational maturity. High-volume practices (>8,000 annual patients) benefit from European brands’ integrated ecosystems and service SLAs. Satellite clinics, startups, and value-focused distributors should evaluate Carejoy for capital-efficient digital adoption—prioritizing verifiable DICOM compliance and regulatory certification over brand legacy. Distributors should position Carejoy as a strategic entry point for clinics transitioning from film, emphasizing TCO advantages while transparently addressing service infrastructure limitations in Tier-2/3 regions.
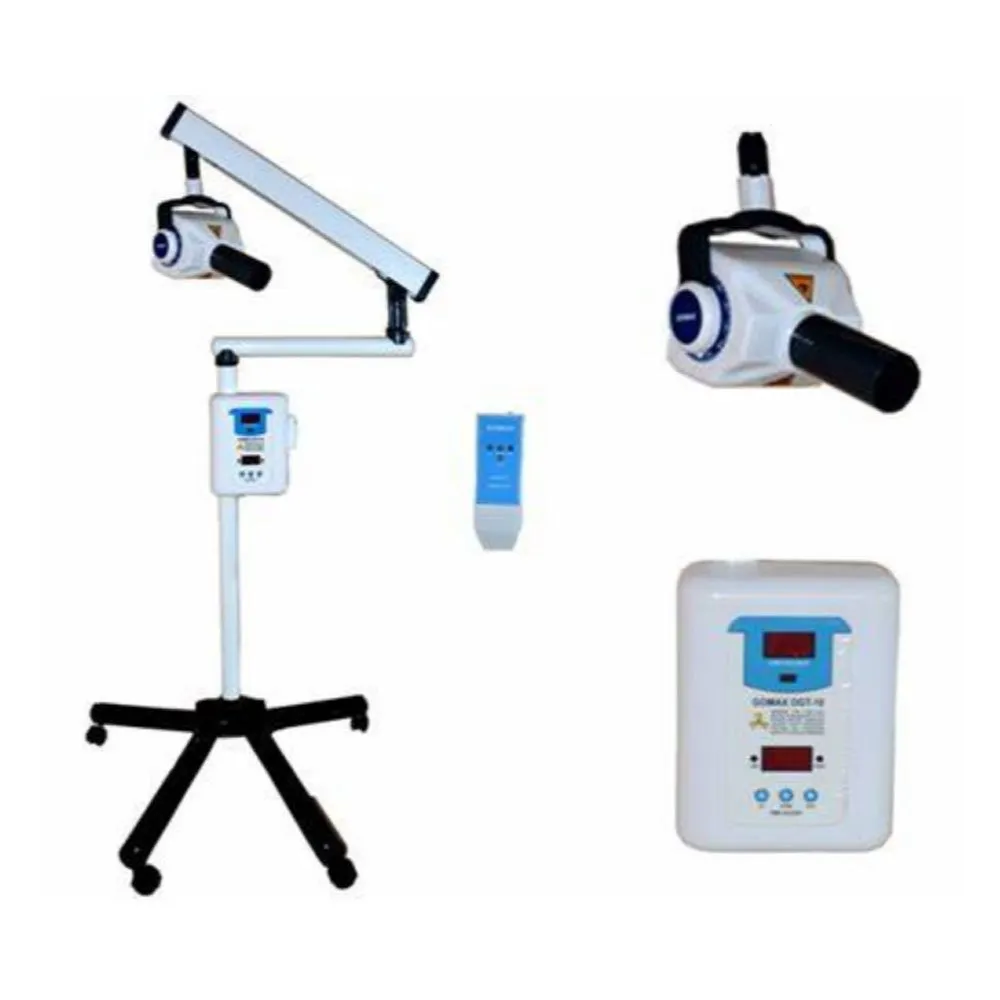
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: iOPA X-Ray Machine Series
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model (iOPA-S100) | Advanced Model (iOPA-A200) |
|---|---|---|
| Power | 70 kVp maximum output, 4 mA tube current, 60 Hz AC input, 110–120 V ±10% power supply. Integrated high-frequency generator for stable exposure control. | 90 kVp maximum output, 8 mA tube current, 50/60 Hz universal input, 100–240 V ±10% auto-switching power supply. Advanced high-frequency inverter with dose optimization algorithms. |
| Dimensions | Height: 1450 mm, Width: 320 mm, Depth: 480 mm. Wall-mounted or floor-stand compatible. Net weight: 28 kg. | Height: 1520 mm (adjustable), Width: 340 mm, Depth: 510 mm. Motorized vertical and horizontal positioning. Net weight: 34 kg with full articulation arm. |
| Precision | ±2° angular accuracy, manual positioning with digital angle readout. Repeatability within 3% across 100 exposures. Compatible with Rinn XCP systems. | ±0.5° angular accuracy with laser-guided alignment. Auto-positioning memory presets for common views (PA, BW, Occlusal). Repeatability within 1% via closed-loop feedback system. |
| Material | Exterior housing: Reinforced ABS polymer with anti-microbial coating. Arm joints: Anodized aluminum alloy. Radiation shielding: Lead-lined collimator (0.8 mm Pb equivalent). | Exterior housing: Medical-grade stainless steel with scratch-resistant finish. Articulating arm: Carbon-fiber composite with titanium joints. Shielding: 1.2 mm Pb equivalent with automatic collimation. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, IEC 60601-1-2 4th Edition (EMC), RoHS certified. | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, IEC 60601-1 & IEC 60601-2-54, IEC 62304 (Software), UL/CSA certified for North America. |
iOPA Dental Imaging Systems – Technical Division | Guide Version: 2026.1 | Contact: [email protected]
Specifications subject to change without notice. For distribution and clinical procurement only.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
i-OPA X-ray Machine Procurement from China
Executive Summary
This 2026 guide provides dental clinics and distributors with a structured methodology for sourcing i-OPA (intraoral panoramic apparatus) X-ray machines from China while mitigating compliance, quality, and logistical risks. With regulatory frameworks evolving globally (including updated EU MDR 2025 Annex XVI requirements and FDA 21 CFR Part 1020.30 revisions), rigorous supplier vetting and contractual precision are non-negotiable. Shanghai Carejoy Medical Co., LTD is highlighted as a pre-vetted manufacturing partner meeting 2026 compliance standards.
Step 1: Verifying ISO/CE Credentials (2026 Compliance Protocol)
Regulatory non-compliance accounts for 68% of rejected dental X-ray shipments (2025 IEC Data). Prioritize suppliers with:
| Credential | 2026 Verification Method | Red Flags | Action Required |
|---|---|---|---|
| ISO 13485:2025 | Validate certificate via IAF CertSearch + request Notified Body audit report | Certificate issued by non-IAF member body | Require full audit trail for radiation safety testing |
| CE Marking (MDR 2025) | Confirm via EU EUDAMED database lookup (NB ID: 0XXX) | Generic “CE” without NB number | Demand EU Technical Documentation dossier |
| FDA 510(k) / Clearance | Verify K-number in FDA Premarket Notifications database | Claims of “FDA Registered” without 510(k) | Require K-number proof for target markets |
| Radiation Safety Cert | Check IEC 60601-2-63:2025 compliance test reports | Absence of leakage dose measurements | Request third-party lab certification (e.g., TÜV) |
Step 2: Negotiating MOQ & Commercial Terms
Chinese manufacturers increasingly enforce strategic MOQs to cover 2026’s elevated component costs (e.g., rare-earth sensors, lead-lined cabinets). Avoid hidden costs through structured negotiation:
| Term | Standard Offer (2026) | Distributor Advantage | Risk Mitigation |
|---|---|---|---|
| Base MOQ | 5-10 units (i-OPA) | Negotiate 3-unit trial order with +15% unit cost | Include penalty clause for non-compliant units |
| Payment Terms | 30% deposit, 70% pre-shipment | Escrow service for balance payment post-verification | Require ISO-certified pre-shipment inspection report |
| OEM Customization | Min. 20 units + $5k setup fee | Waived setup for 50+ unit annual commitment | Specify UI/UX requirements in Appendix A |
| Warranty | 12 months parts/labor | Extend to 24 months with service contract | Define spare parts availability (min. 7 years) |
Step 3: Shipping & Logistics (DDP vs. FOB 2026 Analysis)
With 2026’s volatile freight rates (+22% YoY per Drewry), clarify Incoterms® 2020 responsibilities:
| Term | Cost Control | Compliance Risk | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (avg. $4,200/40ft) | High: Must manage customs clearance, radiation certification | Experienced importers with in-house compliance team |
| DDP [Your City] | Fixed all-in price (20-25% premium) | Low: Supplier bears import duty, VAT, local certification | 90% of new distributors (eliminates port demurrage risk) |
Pre-Vetted Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: ISO 13485:2025 certified (TÜV SÜD Certificate No. Q225 0000582) with active CE MDR 2025 compliance for i-OPA systems (NB ID: 2797)
- MOQ Flexibility: 3-unit trial orders accepted for distributors; OEM setup waived at 40+ units/year
- Logistics: DDP shipping to 87 countries with radiation compliance documentation included
- Quality Assurance: In-house radiation testing lab (CNAS L12895 accredited) + 24-month warranty standard
Direct Sourcing Channel
Company: Shanghai Carejoy Medical Co., LTD
Experience: 19 Years Dental Equipment Manufacturing & Export (Est. 2007)
Location: Baoshan District, Shanghai, China (Factory Direct)
Core Offerings: i-OPA X-ray, Dental Chairs, CBCT, Intraoral Scanners, Autoclaves (OEM/ODM)
Contact: [email protected] | WhatsApp: +86 15951276160
Verification: Request Certificate Package #CJ-2026-DX via email
Conclusion: 2026 Sourcing Imperatives
Successful i-OPA procurement requires moving beyond price-centric negotiations to structured compliance validation. Prioritize suppliers with:
- Active 2026 regulatory certifications (ISO 13485:2025, MDR 2025)
- Transparent radiation safety documentation protocols
- DDP shipping capabilities to mitigate import risks
Shanghai Carejoy’s 19-year export history, CNAS-accredited testing facility, and distributor-focused commercial terms position them as a low-risk sourcing partner for dental clinics and distributors navigating 2026’s complex regulatory landscape.
Disclaimer: This guide reflects 2026 regulatory standards. Verify all credentials with relevant authorities. Prices and terms subject to change based on component market fluctuations.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Frequently Asked Questions – iOpa X-Ray Machine Procurement in 2026
Frequently Asked Questions: iOpa X-Ray Machine Purchase Considerations (2026)
| Question | Professional Insight |
|---|---|
| 1. What input voltage requirements should I verify before purchasing an iOpa X-ray machine in 2026? | iOpa X-ray units typically operate on standard 100–240 V AC, 50/60 Hz, making them compatible with global power systems. However, clinics must confirm local voltage stability and grounding compliance. In regions with inconsistent power supply, integration with a voltage stabilizer or uninterruptible power supply (UPS) is recommended to protect sensitive imaging components and ensure regulatory compliance with IEC 60601-1 safety standards. |
| 2. Are spare parts for iOpa X-ray machines readily available, and what is the typical lead time for critical components? | As of 2026, iOpa maintains an established global spare parts network through authorized distributors and service hubs. Common components such as tube heads, sensors, collimators, and control boards are generally in stock with lead times of 3–7 business days for in-region orders. We recommend clinics and distributors maintain a preventive maintenance inventory of high-wear items. Extended service agreements often include priority access to expedited spare parts logistics. |
| 3. What does the installation process for an iOpa X-ray machine involve, and is on-site technician support included? | Installation of an iOpa X-ray system requires certified biomedical technicians to perform site assessment, wall mounting (if applicable), electrical safety checks, radiation shielding verification, and software calibration. Most 2026 purchase agreements from authorized dealers include complimentary on-site installation and staff training. Remote diagnostic setup and DICOM integration support are also standard. Dental clinics must ensure structural readiness and compliance with local radiation safety regulations prior to installation scheduling. |
| 4. What is the standard warranty coverage for a new iOpa X-ray machine purchased in 2026? | iOpa X-ray machines purchased in 2026 come with a comprehensive 3-year limited warranty covering the X-ray tube, generator, arm mechanics, and control electronics against manufacturing defects. The warranty includes labor and parts replacement when serviced by authorized technicians. Extended warranty options up to 5 years are available and highly recommended for high-volume practices. Note: Warranty is void if non-approved accessories or third-party repairs are used. |
| 5. How does iOpa support clinics and distributors regarding post-warranty service and spare parts pricing? | iOpa offers transparent post-warranty service contracts with tiered support levels (Basic, Premium, Platinum), including priority response times, annual preventive maintenance, and discounted spare parts pricing. Distributors receive dedicated technical support portals, access to firmware updates, and training certifications. Spare parts are priced competitively with volume discounts available for multi-clinic networks and distribution partners under annual service agreements. |
Need a Quote for Iopa X Ray Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


