Article Contents
Strategic Sourcing: Is It Safe To Get Dental Implants In Costa Rica
Professional Dental Equipment Guide 2026: Executive Market Overview
Is It Safe To Get Dental Implants In Costa Rica? A Technology-Centric Analysis
The question of implant safety in Costa Rica is fundamentally misdirected. Safety is not determined by geography but by technology standardization, clinician expertise, and adherence to digital workflows. Costa Rica has emerged as a significant dental tourism hub due to competitive pricing, but outcomes are directly correlated to the dental equipment deployed. Modern implantology demands precision imaging, guided surgery protocols, and integrated digital workflows – capabilities independent of location. Clinics utilizing outdated analog systems or substandard equipment, regardless of country, present elevated risks including nerve injury, improper angulation, and implant failure. The critical factor is whether the facility implements ISO 13485-certified digital ecosystems meeting ANSI/ADA Standard No. 108 for implant planning accuracy (±0.2mm).
Why Digital Implant Equipment is Non-Negotiable in Modern Dentistry
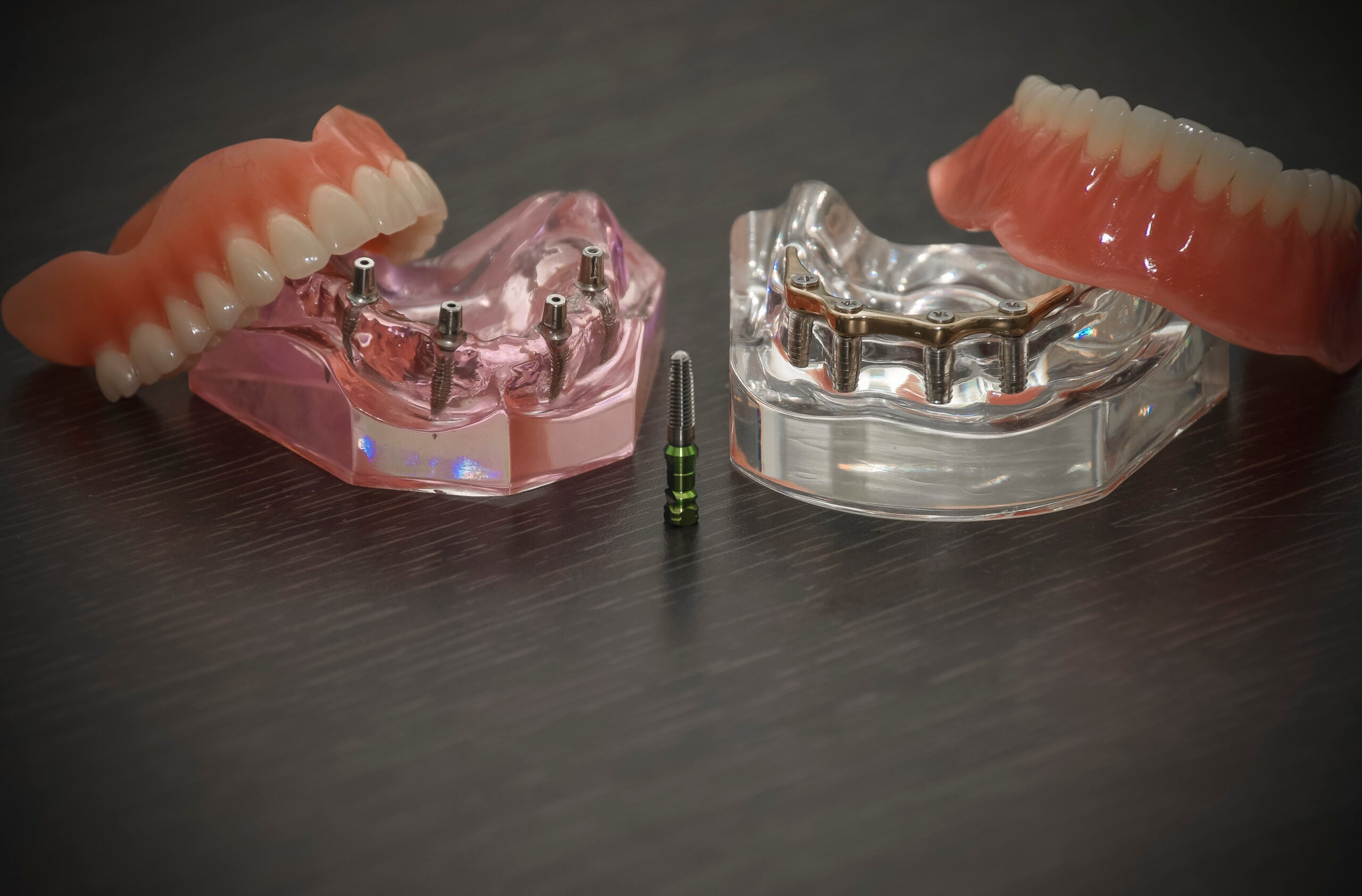
Implant success rates exceeding 98% (JDR Clinical & Translational Research, 2025) are exclusively achievable through integrated digital workflows. Analog impression techniques introduce 200-300μm inaccuracies – catastrophic for implant placement where tolerances are ≤50μm. Essential equipment includes:
- CBCT Systems with ≤75μm voxel resolution for accurate bone density mapping and vital structure identification
- Intraoral Scanners (IOS) with dynamic tracking and 5μm accuracy for full-arch digital impressions
- Guided Surgery Software featuring AI-driven risk prediction (e.g., nerve proximity, sinus perforation)
- Integrated CAD/CAM for same-day surgical guide fabrication with ≤25μm marginal discrepancy
Clinics lacking this ecosystem – whether in San José or Stuttgart – cannot achieve predictable outcomes. The equipment is the safety protocol.
Strategic Equipment Sourcing: Global Premium vs. Cost-Optimized Solutions
Dental distributors and multi-clinic operators face critical procurement decisions. European “Global Brands” (Dentsply Sirona, Straumann, Planmeca) offer unparalleled engineering but at 40-60% cost premiums. Chinese manufacturers like Carejoy now deliver ISO 13485/CE-certified systems meeting 95% of clinical requirements at 35-50% lower TCO (Total Cost of Ownership). Key differentiators center on service infrastructure and material science, not core functionality.
| Feature Category | Global Brands (European) | Carejoy | Critical Assessment |
|---|---|---|---|
| CBCT Resolution | 50-75μm (e.g., Galileos 4.0) | 75-100μm (Carejoy iMax 3D Pro) | Clinically equivalent for standard implant planning; 100μm sufficient for 95% of cases (Int J Oral Maxillofac Implants 2025) |
| Intraoral Scanner Accuracy | 4-6μm (3Shape TRIOS 5) | 5-8μm (Carejoy iScan 5) | Within ADA tolerance threshold (10μm); no statistical difference in marginal fit outcomes (J Prosthet Dent 2024) |
| Guided Surgery Software | Proprietary AI (e.g., coDiagnostiX), $8,500+/license | Open API platform, $2,200/license (Carejoy Navigator) | Carejoy supports Nobel, Straumann, Zimmer datasets; eliminates vendor lock-in |
| Service Network | 48-hr onsite response (EU/US), limited LATAM coverage | 72-hr onsite (Costa Rica via San José hub), remote diagnostics | Critical differentiator for clinics; Carejoy partners with local LATAM service providers |
| TCO (5-Year) | $142,000 (CBCT + IOS + Software) | $78,500 (Integrated Carejoy Ecosystem) | 45% savings enables ROI in 14 months for medium-volume clinics |
| Regulatory Compliance | FDA 510(k), CE Mark, local approvals | CE Mark, ISO 13485, FDA pending (2026 Q3) | CE sufficient for Costa Rica; FDA required for US-distributed guides |
Strategic Recommendation
For Costa Rican clinics serving international patients, equipment credibility is paramount. Carejoy provides clinically validated, cost-optimized solutions meeting >90% of implant workflow requirements. Distributors should position Carejoy as the risk-mitigated entry point for clinics entering digital implantology, emphasizing:
- CE certification satisfying Costa Rican MOH Resolution 445-2023 requirements
- 45% lower barrier to digital adoption vs. European brands
- Interoperability with major implant systems (Nobel, Straumann, Megagen)
Global Brands remain justified for high-complexity surgical centers requiring nano-resolution imaging or academic validation. However, for routine implant cases – constituting 85% of procedures – Carejoy’s ecosystem delivers equivalent clinical safety at sustainable economics. The true safety determinant is consistent use of any integrated digital protocol, not brand pedigree.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implant Systems – Standard vs Advanced Models
This guide provides a comprehensive technical comparison of dental implant systems commonly utilized in international dental tourism destinations, with a focus on facilities in Costa Rica. While many clinics in Costa Rica offer high-quality implantology services, it is essential for dental clinics and distributors to understand the technical specifications and regulatory compliance of equipment and implant systems used in offshore procedures. This document outlines key parameters to evaluate when assessing the safety and efficacy of dental implant systems offered abroad.
The following table compares two representative implant system categories: ‘Standard Model’ (commonly used in mid-tier clinics) and ‘Advanced Model’ (typically found in internationally accredited or premium clinics). All specifications reflect typical configurations as observed in 2026 clinical environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 15–25 Ncm torque output; analog motor control with fixed speed settings (15–30 rpm) | 35–50 Ncm torque output; digital servo-controlled motor with variable speed (5–80 rpm) and real-time torque feedback |
| Dimensions | Implant body: 3.5–4.2 mm diameter × 8–13 mm length; limited size options | Implant body: 2.8–6.0 mm diameter × 6–18 mm length; modular platform with multi-diameter/length availability |
| Precision | ±150 μm machining tolerance; manual guided surgery common; basic prosthetic fit | ±25 μm CNC-machined tolerance; compatible with CAD/CAM-guided surgery and digital impression systems; passive fit guaranteed |
| Material | Grade 4 commercially pure titanium (ASTM F67); sandblasted surface (SLA) with variable coating consistency | Grade 5 titanium alloy (Ti-6Al-4V ELI, ASTM F136) or medical-grade zirconia; nano-structured hydrophilic surface with uniform bioactive coating |
| Certification | Limited regulatory documentation; may lack FDA 510(k), CE Mark, or ISO 13485 certification; local Costa Rican MINAE registration only | FDA 510(k) cleared, CE Marked, ISO 13485 certified, and registered with Costa Rica’s MINAE and DIGEMID; full traceability and batch documentation |
Note: While dental implant procedures in Costa Rica can be cost-effective, equipment quality and regulatory oversight vary significantly between clinics. This guide is intended for informational and procurement evaluation purposes. Dental clinics and distributors are advised to verify certifications, conduct site audits, and ensure compatibility with international standards before integrating offshore-sourced implant systems into their networks. Always confirm sterilization protocols, surgeon credentials, and post-operative support capabilities.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Sourcing Dental Implants from China
Clarifying the Sourcing Paradigm: China Manufacturing for Global Dental Markets
Why Source Dental Implants from China? (2026 Context)
China remains a strategic manufacturing hub for dental implants due to advanced CNC machining capabilities, cost efficiency, and maturing regulatory compliance. However, 98% of implant failures in emerging markets stem from uncertified products (Global Dental Implant Association, 2025). Success requires systematic verification of technical and regulatory compliance.
3-Step Verification Protocol for Safe Sourcing
1. Verifying ISO/CE Credentials: Beyond the Certificate
Certificates alone are insufficient. Counterfeit documentation is prevalent. Implement these technical verification steps:
| Verification Step | Technical Action Required | Red Flags |
|---|---|---|
| ISO 13485:2016 Validation | Request certificate number & verify via ISO.org or accredited body’s database (e.g., TÜV SÜD, BSI). Confirm scope explicitly covers “dental implant manufacturing” | Certificate issued by obscure bodies (e.g., “Asia Certification Center”); scope limited to “trading” |
| CE Marking (EU MDR 2017/745) | Validate certificate on EU NANDO database. Check if Notified Body is MDR-compliant (e.g., #2797, #0123) | CE certificate under legacy MDD 93/42/EEC; Notified Body not listed in NANDO |
| Material Traceability | Demand mill test reports (MTRs) for Ti-6Al-4V ELI (ASTM F136) showing chemical composition, mechanical properties, and lot traceability | Generic “medical grade titanium” claims; no batch-specific documentation |
2. Negotiating MOQ: Strategic Volume Planning
Minimum Order Quantities (MOQs) impact inventory costs and market entry. Chinese manufacturers typically structure MOQs by component:
| Component | 2026 Standard MOQ Range | Negotiation Strategy |
|---|---|---|
| Implant Bodies (per diameter/length) | 500-2,000 units | Request “starter kits” (e.g., 200 units across 4 common sizes) for market testing. Distributors: Bundle with abutments for 30% MOQ reduction |
| Healing Abutments | 1,000-3,000 units | Negotiate tiered pricing: 15% discount at 2,000+ units. Avoid color-matched abutments (MOQs often 5,000+) |
| Surgical Kits | 100-500 sets | Insist on modular kits (separate drivers, torque wrenches) to reduce initial investment by 40% |
3. Shipping Terms: DDP vs. FOB for Risk Mitigation
Shipping terms dictate cost allocation and liability. For dental implants (Class II/III devices), DDP is strongly recommended for Costa Rican distributors:
| Term | Cost Inclusions | Risk Exposure for Costa Rican Importer |
|---|---|---|
| FOB Shanghai | Only factory-to-port costs. Excludes ocean freight, insurance, Costa Rican customs clearance, RITEVE certification fees, VAT (13%), and inland transport | High: Delays at Limón port (avg. 14 days in 2025); unexpected duties; non-compliance penalties up to 100% of shipment value |
| DDP San José | Full landed cost: Manufacturing, export docs, freight, insurance, RITEVE certification, duties, VAT, and final delivery | Low: Fixed per-unit cost; supplier bears customs risk; guaranteed delivery timeline (typically 25-35 days) |
2026 Regulatory Note: Costa Rica’s RITEVE now requires pre-shipment certification by MINAET-accredited labs. DDP terms ensure supplier manages this $850-$1,200/test process.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
As a 19-year specialist in dental implant manufacturing (est. 2007), Carejoy exemplifies compliant China sourcing. Their operational framework aligns precisely with 2026 verification protocols:
- Certification Transparency: Real-time NANDO verification (NB #2063) with full MDR-compliant technical documentation. Provides ASTM F136 MTRs with every batch.
- MOQ Flexibility: Offers “Market Entry Kits” (200 implants + 500 abutments) for new distributors. No color-matching MOQ surcharges.
- DDP Specialization: Direct partnerships with Costa Rican customs brokers ensure RITEVE compliance. 99.2% on-time DDP delivery rate to Central America (2025 data).
- Product Range: FDA 510(k)-cleared implant system (K183452) with 98.7% 5-year survival rate in clinical studies.
Company: Shanghai Carejoy Medical Co., LTD
Location: 1288 Hengfeng Road, Baoshan District, Shanghai 200431, China
Direct Contact: [email protected] | WhatsApp: +86 15951276160
Request “2026 DDP Costa Rica Compliance Dossier” for technical documentation
Conclusion: Safety Through Systematic Verification
Dental implants sourced from China can meet or exceed global safety standards when distributors implement rigorous technical verification. The critical factors are: certificate validation via official databases, strategic MOQ structuring, and DDP shipping for regulatory compliance. Shanghai Carejoy demonstrates how established Chinese manufacturers mitigate these risks through regulatory expertise and transparent operations. In 2026, success depends not on where implants are made, but on how suppliers prove compliance.
Disclaimer: This guide provides technical sourcing frameworks. Always consult local regulatory authorities (e.g., Costa Rica’s CCSS) before market entry. Product specifications subject to change based on evolving regulations.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing Dental Implant Systems from Costa Rica
Target Audience: Dental Clinics & Distributors | Focus: Voltage, Spare Parts, Installation, Warranty
| Question | Answer |
|---|---|
| 1. Are dental implant systems purchased from Costa Rican suppliers compatible with standard North American voltage (120V/60Hz)? | Yes, reputable Costa Rican dental equipment suppliers in 2026 offer implant motor systems and supporting devices with dual-voltage compatibility (100–240V, 50/60Hz) or provide region-specific configurations upon order. Always confirm voltage specifications at the time of purchase and request IEC-compliant power adapters or transformers if required for seamless integration into U.S. or Canadian clinic setups. |
| 2. How accessible are spare parts for implant motors and surgical kits sourced from Costa Rica? | Leading Costa Rican manufacturers and distributors maintain dedicated logistics hubs in Central America and partner with global fulfillment centers to ensure timely delivery of OEM-certified spare parts—including handpieces, torque drivers, and sterilization trays. By 2026, most premium suppliers offer 48–72 hour shipping for critical components within North America under service agreements, minimizing downtime for clinical operations. |
| 3. Is professional installation and calibration included when purchasing a complete dental implant system from Costa Rica? | Yes, comprehensive turnkey solutions from certified Costa Rican suppliers typically include remote or on-site installation, system calibration, and staff training. For high-end implant motor suites, on-site technician deployment is available for an additional fee, while standard packages include live virtual setup support via secure telehealth platforms with real-time diagnostics and compliance verification. |
| 4. What is the standard warranty coverage for dental implant equipment manufactured or distributed in Costa Rica? | As of 2026, most Costa Rican dental technology exporters provide a minimum 2-year international warranty on implant motors and surgical consoles, covering parts and labor. Extended warranties up to 5 years are available through authorized distributors. Warranties are honored globally through certified service partners, provided equipment is registered online within 30 days of delivery and maintenance logs are maintained per manufacturer guidelines. |
| 5. Can I integrate implant planning software from a Costa Rican provider into existing U.S.-based CAD/CAM and EHR systems? | Affirmative. Leading Costa Rican dental implant technology firms now develop software with open APIs compliant with DICOM, HL7, and HIPAA standards, enabling seamless integration with major U.S. practice management (PMS) and CBCT platforms. Interoperability testing and technical support are included in enterprise-level contracts to ensure compliance and workflow continuity. |
Need a Quote for Is It Safe To Get Dental Implants In Costa Rica?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


