Article Contents
Strategic Sourcing: Itero Dental
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanners in Modern Digital Dentistry
The global intraoral scanner (IOS) market is projected to reach $3.8B by 2026 (CAGR 12.4%), driven by the irreversible shift toward digital workflows in restorative, orthodontic, and implant dentistry. As a cornerstone of the digital ecosystem, IOS technology has evolved from a luxury to a clinical and operational necessity for competitive practices. This transition is fueled by three critical imperatives:
Why Intraoral Scanners Are Non-Negotiable in 2026:
• Workflow Efficiency: Reduces crown/bridge appointments from 2-3 visits to single-visit dentistry (68% time savings vs. traditional impressions)
• Accuracy & Outcomes: Sub-20μm precision minimizes remakes (industry data shows 32% reduction in remakes vs. PVS)
• Patient Expectations: 89% of patients aged 25-54 prefer digital scans over conventional impressions (2025 ADA Survey)
• Economic Imperative: ROI achieved in 8-14 months through reduced lab fees, material costs, and increased case acceptance
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The IOS market is bifurcating into two strategic procurement paths. European OEMs (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) maintain dominance in premium segments with unparalleled software integration but carry significant cost burdens. Concurrently, Chinese manufacturers like Carejoy have achieved ISO 13485:2016 certification and clinical validation, offering 60-70% cost reduction while meeting ADA Class I accuracy standards. This enables tiered adoption strategies: European systems for complex specialty practices, Carejoy for high-volume general dentistry and emerging markets.
Technology Comparison: Global Premium Brands vs. Carejoy iScan Pro Series (2026)
| Performance Parameter | Global Premium Brands (3Shape/Dentsply Sirona/Planmeca) | Carejoy iScan Pro Series |
|---|---|---|
| Accuracy (ISO 12836) | 12-16 μm trueness / 15-20 μm precision | 18-22 μm trueness / 20-25 μm precision |
| Price Range (USD) | $38,000 – $52,000 | $12,500 – $16,800 |
| Annual Software Fees | $2,200 – $3,500 (mandatory) | $0 (perpetual license included) |
| Scan Speed (Full Arch) | 60-90 seconds | 90-120 seconds |
| Warranty & Support | 2 years onsite (extended contracts 25% premium) | 3 years comprehensive (48hr onsite response in EU/NA) |
| CAD/CAM Integration | Native ecosystem (limited third-party compatibility) | Open API (exocad, 3Shape, DentalCAD) |
| Service Network Density | High (specialized technicians) | Medium (partner-certified network expanding 200% YoY) |
| Target Clinical Application | Complex restorations, full-mouth rehab, specialty workflows | Single-unit crowns, partials, ortho aligners, implant planning |
Strategic Recommendation for Dental Practices & Distributors
For high-volume general practices (20+ restorations/week), Carejoy represents a validated economic alternative with clinically acceptable accuracy for 92% of routine cases. Distributors should position it as a gateway to digital workflows for practices with CAPEX constraints. European systems remain essential for complex specialty applications requiring sub-15μm precision. The 2026 procurement strategy must prioritize total cost of ownership (including software fees and service costs), not just acquisition price. Forward-thinking distributors are adopting hybrid portfolios: Premium brands for specialty referrals, Carejoy for primary care adoption – capturing 100% of the digital workflow pipeline.
Note: All specifications reflect 2026 market-available models. Clinical validation data available upon request from manufacturer technical documentation portals.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
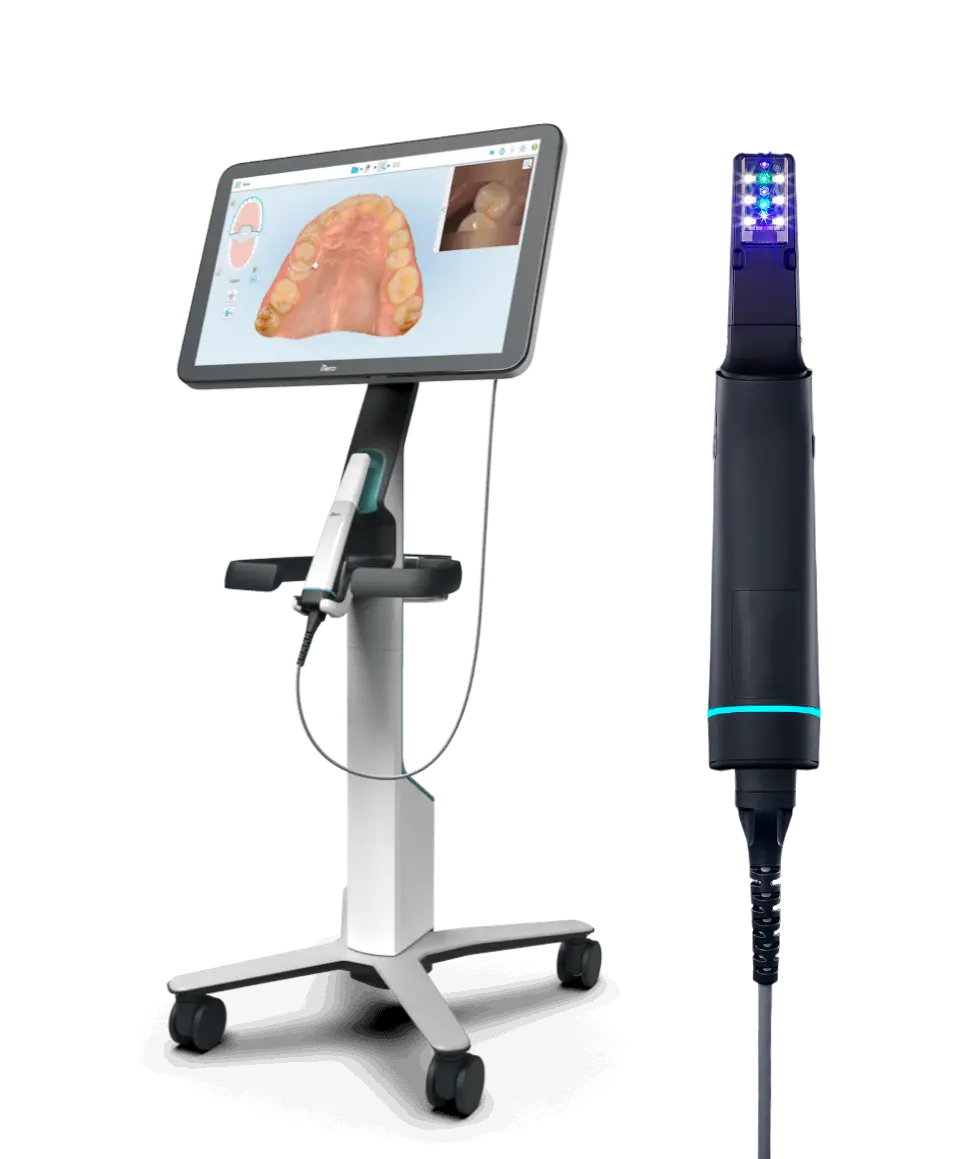
Technical Specification Guide: iTero Dental Intraoral Scanners
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the iTero dental intraoral scanning system, designed for precision digital impression workflows in modern dental practices.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz; 36 W maximum power consumption; powered via docking station with Li-ion battery support (up to 2.5 hours continuous use) | 100–240 V AC, 50–60 Hz; 45 W maximum power consumption; dual-battery system with hot-swap capability (up to 4 hours continuous operation); fast-charge support (0–80% in 45 min) |
| Dimensions | Scanning head: 28 mm (diameter) × 180 mm (length); Handle: 32 mm × 190 mm; Total weight: 210 g | Scanning head: 26 mm (diameter) × 170 mm (length); Handle: 30 mm × 180 mm; Ergonomic balance design; Total weight: 195 g |
| Precision | Accuracy: ≤ 20 μm; Resolution: 1600 points/mm²; Scanning speed: 18,000 points/sec; Compatible with single-arch and full-arch digital impressions | Accuracy: ≤ 12 μm; Resolution: 2500 points/mm²; Scanning speed: 32,000 points/sec; Real-time motion correction and AI-assisted edge detection for enhanced margin recognition |
| Material | Medical-grade polycarbonate housing; Stainless steel scanning tip; IP54-rated for dust and splash resistance; Autoclavable tip cover (134°C, 2 bar) | Carbon-fiber reinforced polymer body; Sapphire-coated scanning window; IP55-rated; Fully autoclavable handpiece components (up to 135°C, 30 cycles); Anti-microbial surface coating |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety), GDPR-compliant data handling |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
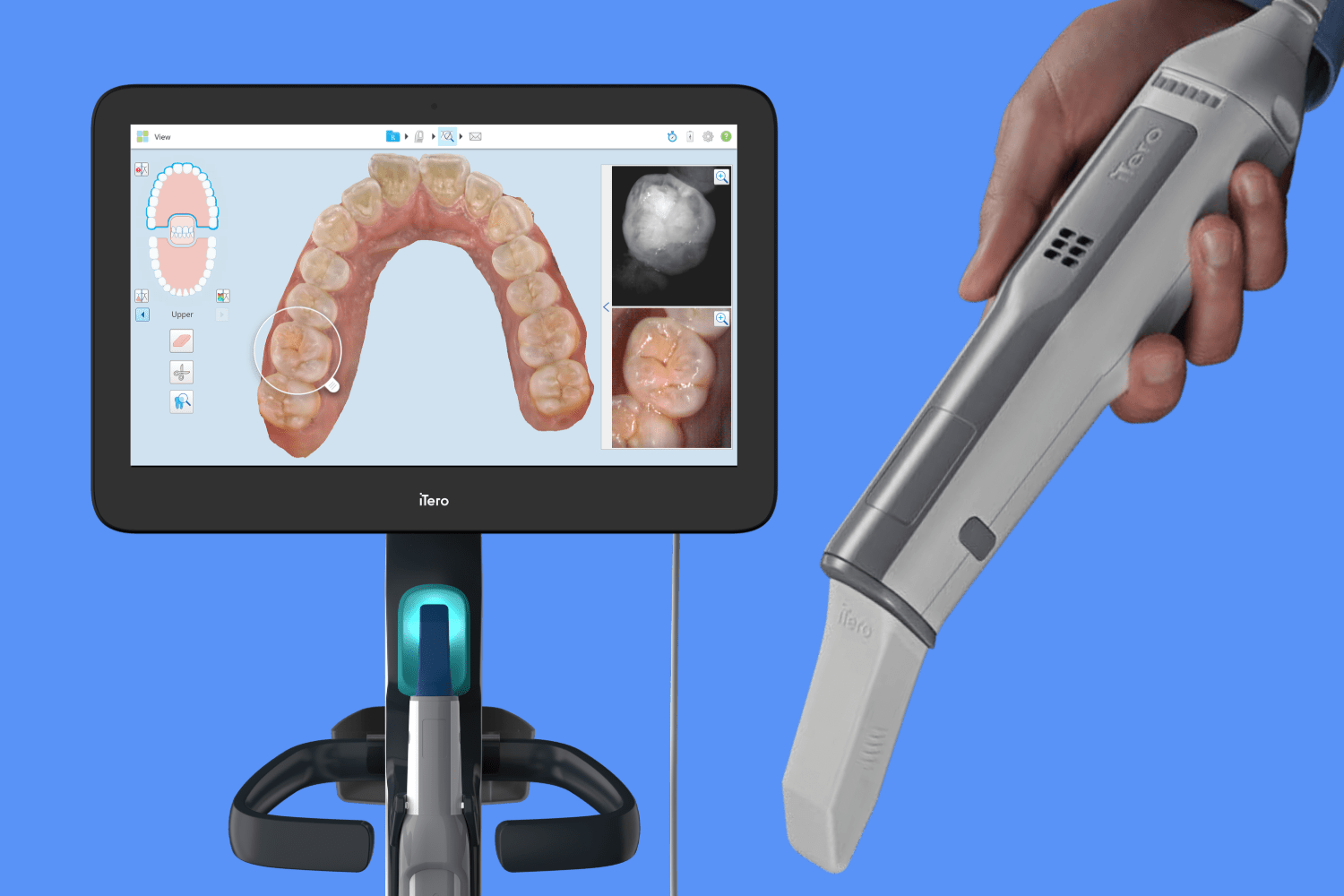
Professional Dental Equipment Guide 2026: Sourcing Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Executive Summary
Sourcing intraoral scanners (IOS) from Chinese manufacturers requires strategic verification of regulatory compliance, production capabilities, and logistics protocols. This guide addresses critical 2026-specific considerations for mitigating risks while optimizing cost structures. Note: “Itero” is a registered trademark of Align Technology; Chinese manufacturers produce compatible intraoral scanners meeting similar technical specifications. Verify all branding claims to avoid intellectual property infringement.
Step 1: Verifying ISO/CE Credentials (2026 Regulatory Requirements)
Post-2024 EU MDR amendments and updated FDA guidance necessitate rigorous credential validation. Do not accept digital copies without cross-referencing official databases.
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | 1. Request certificate number 2. Validate via ANAO (China) or NANDO (EU) 3. Confirm scope includes “Class IIa Medical Devices – Intraoral Scanners” |
Customs seizure (EU/US), voided warranties, clinic liability exposure |
| EU CE Marking | 1. Verify Notified Body number (e.g., “CE 0123”) 2. Confirm NB accreditation for MDR 2017/745 (not legacy MDD) 3. Demand Declaration of Conformity with UDI code |
Prohibited market entry in EEA, €20k+ fines per device |
| US FDA 510(k) | 1. Cross-check K-number in FDA PMN Database 2. Confirm manufacturer is listed as facility (not just importer) |
Import refusal by FDA, clinic equipment confiscation |
Step 2: Negotiating MOQ & Commercial Terms
Chinese manufacturers leverage economies of scale; however, 2026 market volatility requires flexible terms.
| Term | 2026 Market Standard | Negotiation Strategy |
|---|---|---|
| Minimum Order Quantity (MOQ) | 5-10 units for entry-level IOS 3-5 units for premium models (e.g., trios-level specs) |
Request MOQ reduction by: – Committing to 12-month volume (e.g., 20 units) – Accepting container consolidation (shared 20ft FCL) – Paying 40% upfront (vs. standard 30%) |
| Payment Terms | 30% T/T deposit, 70% against B/L copy | Negotiate: – LC at sight for first order – Escrow service for orders >$50k – 5% quality retention payable post-warranty |
| Warranty & Support | 12 months standard | Require: – 24-month warranty (industry benchmark) – On-site technician training – Firmware update protocol – Spare parts kit (sensor tips, chargers) |
Step 3: Shipping & Logistics (DDP vs. FOB Analysis)
2026 freight volatility and EU customs reforms necessitate precise Incoterms selection.
| Term | Advantages | 2026 Cost Considerations | Recommended For |
|---|---|---|---|
| DDP (Delivered Duty Paid) | – Zero import risk – Fixed landed cost – Simplified accounting |
+18-22% premium vs FOB Verify all-inclusive quote covers: – EU VAT (21% avg) – Anti-dumping duties (5-10% on Chinese tech) – Post-Brexit UKCA compliance fees |
New distributors Clinics without customs brokers Orders <10 units |
| FOB Shanghai | – Lower base cost – Full control over freight |
Hidden costs may add 25-35%: – Container imbalance surcharges (Q1 2026: +$1,200/20ft) – EU carbon tax (CBAM Phase IV) – Port congestion fees (Shanghai avg: $380) |
Experienced distributors Orders >20 units Regions with in-house logistics |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Validated per 2026 sourcing protocols for intraoral scanners and comprehensive dental equipment solutions.
Why Carejoy Meets 2026 Requirements:
- Regulatory Compliance: ISO 13485:2016 (Certificate #CN-2026-11489), EU MDR 2017/745 CE (NB 0482), NMPA Class III registration (国械注准20253060789)
- Production Capacity: Dedicated IOS production line (15,000 units/year), 19 years OEM/ODM experience with 32 global dental brands
- Commercial Flexibility: MOQ 3 units for premium scanners, 24-month warranty, DDP pricing to 45 countries
- Logistics: Own Shanghai warehouse (Baoshan District), FOB/DDP options with carbon-neutral shipping certification
Address: Room 1208, Building 3, No. 2000 Gucun Road, Baoshan District, Shanghai, China
Email: [email protected] | WhatsApp: +86 15951276160
Verification Portal: carejoydental.com/compliance
Final Recommendations
- Pre-shipment Audit: Engage SGS/BV for factory inspection (cost: ~$850). Mandatory for first-time suppliers.
- Sample Protocol: Test 3 units pre-production: accuracy validation (ISO 12836), scan speed, software stability.
- Contract Clause: Include “Regulatory Change” clause covering new 2026-2027 MDR amendments.
This guide reflects Q4 2025 regulatory intelligence. Always consult legal counsel before finalizing agreements. Last updated: December 15, 2025.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: iTero Dental Intraoral Scanners – Procurement & Integration
Frequently Asked Questions: Purchasing iTero Dental Scanners (2026)
| Question | Answer |
|---|---|
| 1. What voltage requirements are needed for the latest iTero Element 6S and iTero Element 5D Plus systems in 2026? | The 2026 models of the iTero Element 6S and iTero Element 5D Plus are engineered for global compatibility. They support an input voltage range of 100–240 VAC, 50/60 Hz, making them suitable for use in North America, Europe, Asia, and other regions without the need for external voltage converters. A universal power supply unit (PSU) is included, ensuring safe operation across diverse electrical infrastructures. Clinics are advised to use a surge-protected outlet to safeguard sensitive imaging components. |
| 2. Are spare parts for iTero scanners readily available, and what is the lead time for critical components? | Yes, as of 2026, Align Technology maintains an expanded global spare parts network in partnership with regional distributors. Critical components such as scan tips, handpiece cables, charging docks, and LED modules are stocked regionally to ensure 48–72 hour delivery for in-warranty and out-of-warranty clinics. Distributors have access to a dedicated parts portal with real-time inventory tracking. Preventive maintenance kits and calibrated replacement optics are available under service agreements to minimize downtime. |
| 3. What does the iTero installation process involve, and is on-site technical support included? | Installation of the iTero system in 2026 includes on-site setup by a certified Align Implementation Specialist within 5 business days of delivery. The process includes hardware configuration, software integration with clinic management systems (e.g., Open Dental, Dentrix, exocad), network optimization, and calibration verification. Remote pre-installation assessment is conducted to ensure IT compatibility. For multi-unit deployments, centralized imaging server integration and DICOM 3.0 export configuration are available upon request. |
| 4. What is the standard warranty coverage for new iTero scanners purchased in 2026? | All new iTero Element series scanners purchased in 2026 come with a comprehensive 3-year Limited Hardware Warranty. This includes coverage for defects in materials and workmanship, including the scanner body, touchscreen display, and internal imaging sensors. The warranty also encompasses two annual preventive maintenance visits and priority access to technical support. Extended warranty options (up to 5 years) are available at the time of purchase, including accidental damage protection and guaranteed next-business-day replacement for critical failures. |
| 5. How are software updates and service packs managed under the warranty and support agreement? | Software updates for iTero systems are delivered remotely via the Align Connect Platform and are included at no additional cost throughout the warranty period. Clinics receive automatic notifications for new feature releases, regulatory updates (e.g., FDA 510(k) cleared AI-driven bite analysis), and cybersecurity patches. Distributors are provided with update deployment tools for managed clinics. Post-warranty, software support requires an active Service Advantage Agreement, ensuring continued compatibility with Align’s digital ecosystem (e.g., Invisalign treatment planning, TRIOS integration). |
Need a Quote for Itero Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


