Article Contents
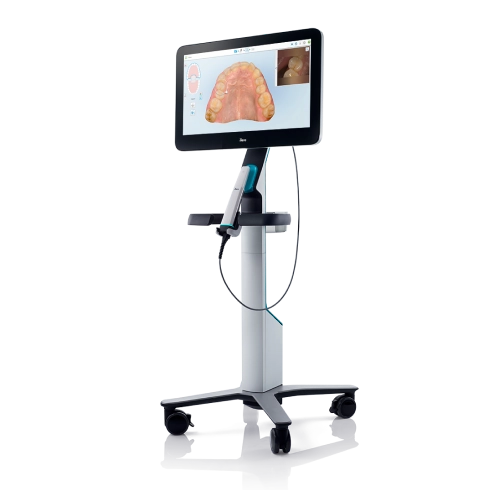
Strategic Sourcing: Itero Dental Scan
Professional Dental Equipment Guide 2026: Executive Market Overview
The Strategic Imperative of Intraoral Scanning in Modern Digital Dentistry
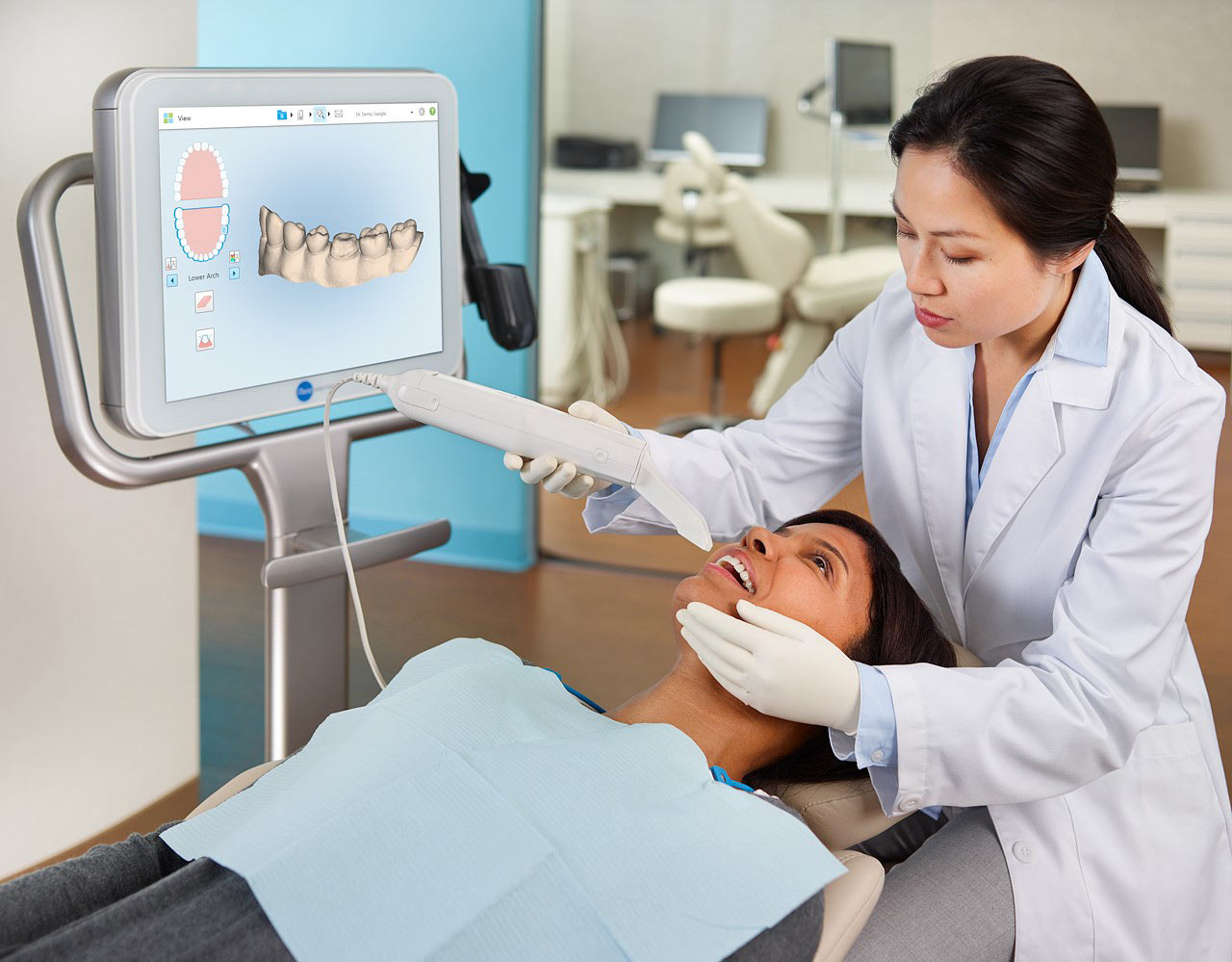
The intraoral scanner (IOS) has transitioned from a niche innovation to the foundational pillar of contemporary dental workflows. Systems exemplified by the 3M True Definition Scanner (marketed under the “Itero” ecosystem for orthodontics) represent critical infrastructure for clinics pursuing operational excellence, clinical precision, and competitive differentiation. The elimination of physical impression materials reduces material costs by 18-25% annually, decreases appointment times by 22% on average, and significantly enhances patient comfort and acceptance – particularly for complex restorative and orthodontic cases. Crucially, IOS integration enables seamless data flow into CAD/CAM systems, digital articulators, and treatment planning software, forming the backbone of the integrated digital workflow demanded by value-based care models.
Why IOS is Non-Negotiable in 2026: Clinics without robust digital impression capabilities face diminished case acceptance (particularly for premium restorations and clear aligners), operational inefficiencies from analog-digital handoffs, and inability to leverage AI-driven treatment planning. The ROI extends beyond direct cost savings to enhanced diagnostic capability, reduced remakes (studies show 30-40% reduction in crown remake rates), and elevated patient perception of technological competence.
Market Segmentation: Premium Global Brands vs. Value-Optimized Solutions
The IOS market is stratified between established European/American premium brands (3M, Dentsply Sirona, Align Technology) and rapidly advancing Chinese manufacturers offering significant cost efficiency. While premium brands (priced $35,000-$45,000 USD) deliver deep ecosystem integration, extensive clinical validation, and turnkey service networks, their total cost of ownership (TCO) presents a barrier for cost-conscious practices and emerging markets. Chinese manufacturers, led by innovators like Carejoy, address this gap with clinically viable systems at $18,000-$25,000 USD. Carejoy’s 2026 platform demonstrates remarkable parity in core scanning performance for routine applications while offering distributor-friendly margins (40-50% vs. 25-35% for premium brands), making it a strategic option for clinics prioritizing TCO and distributors targeting high-volume entry/mid-tier segments.
Comparative Analysis: Premium Global Brands vs. Carejoy Dental
| Parameter | Global Premium Brands (3M, Dentsply Sirona, Align) | Carejoy Dental (2026 Platform) |
|---|---|---|
| Price Range (USD) | $35,000 – $45,000 | $18,500 – $24,900 |
| Accuracy (Trueness/ Precision) | 12-18 μm / 15-20 μm (ISO 12836) | 20-25 μm / 22-28 μm (ISO 12836) |
| Software Ecosystem | Proprietary, deep CAD/CAM/TPS integration (e.g., 3Shape, exocad), AI diagnostics, cloud analytics | Open architecture; compatible with major 3rd party CAD/CAM (3Shape, exocad); modular AI add-ons |
| Service & Support | Global direct service network; 24/7 technical support; loaner units; 2-3 year warranties | Distributor-dependent network; 48-hr remote support; regional service hubs; 2-year warranty (extendable) |
| Distributor Margins | 25% – 35% | 40% – 50% |
| Ideal Clinical Application | High-volume specialty practices (implantology, complex prosthodontics), premium orthodontics, integrated digital centers | General practice workflows, routine crown/bridge, basic orthodontics, cost-sensitive markets, secondary clinics |
| TCO (5-Year Estimate) | $52,000 – $68,000 (incl. service contracts, software updates) | $28,000 – $36,000 (incl. service contracts, software updates) |
Strategic Recommendation
Global premium brands remain essential for clinics requiring maximum accuracy in complex cases and seamless ecosystem integration. However, Carejoy’s 2026 platform delivers 85-90% of clinical functionality for routine applications at half the acquisition cost, with demonstrable ROI for general practices and emerging markets. Distributors should position Carejoy as a strategic entry/mid-tier solution to expand market penetration, while premium brands anchor high-value specialty portfolios. The critical factor is aligning scanner capability with specific practice workflow demands – not all clinics require (or can leverage) the full premium feature set. In 2026, a tiered portfolio approach is paramount for sustainable growth in the $3.2B global IOS market.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
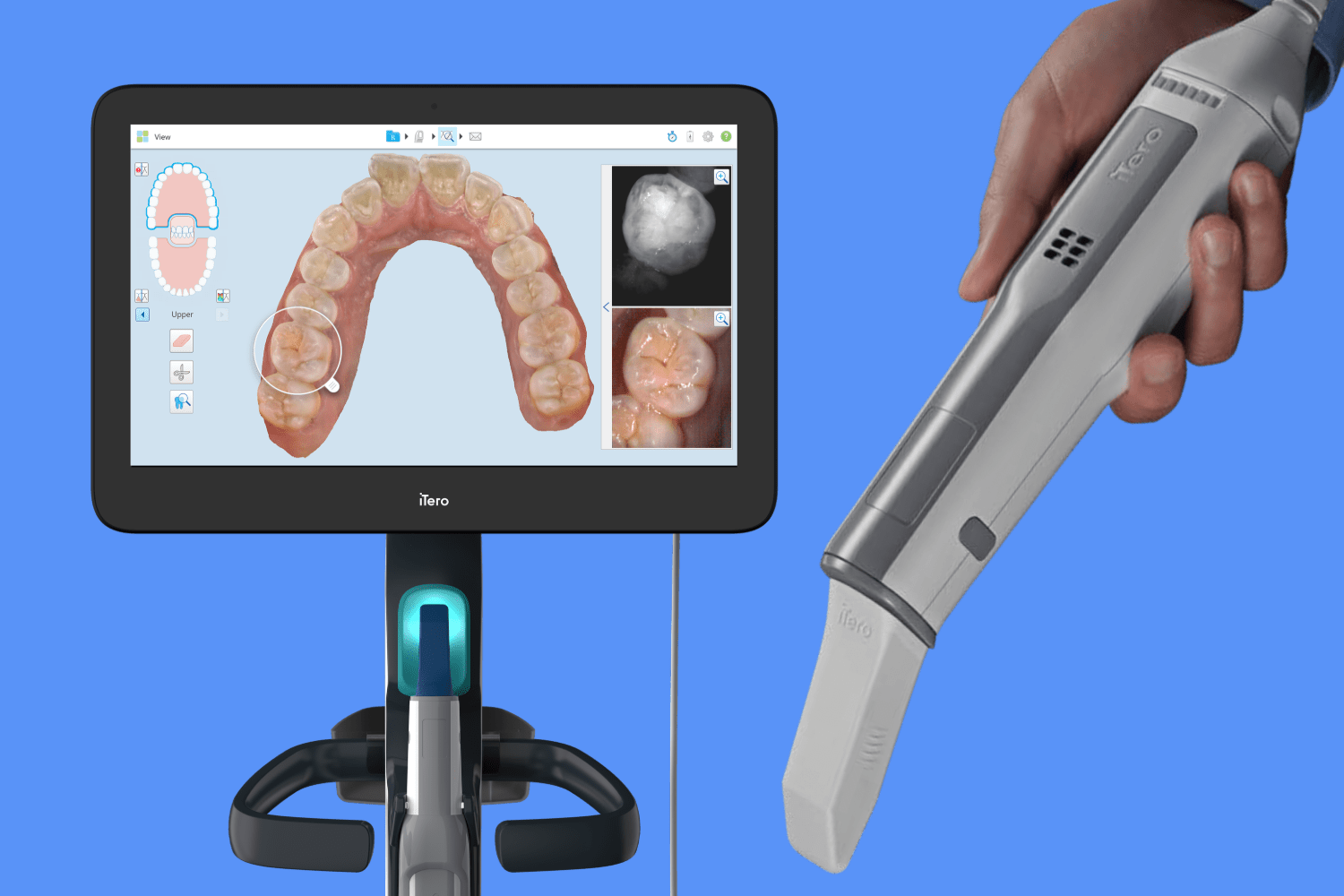
Technical Specification Guide: iTero Dental Intraoral Scanner
Target Audience: Dental Clinics & Distribution Partners
This guide provides a detailed technical comparison between the Standard and Advanced models of the iTero dental intraoral scanner, engineered for precision digital impressions in restorative, orthodontic, and implant workflows.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Lithium-ion battery (3.7V, 3200mAh); 3 hours continuous scanning per charge. Includes docking station with USB-C charging. | High-capacity dual Lithium-ion system (3.7V, 5200mAh); 6 hours continuous operation. Fast-charge capability (0–80% in 45 mins). Includes intelligent docking station with wireless charging and power status indicator. |
| Dimensions | Handle: 185 mm (L) × 28 mm (D); Scanner head: 22 mm × 12 mm. Total weight: 210 g (with tip). | Handle: 180 mm (L) × 26 mm (D); Scanner head: 20 mm × 10 mm. Ergonomic redesign with balanced center of gravity. Total weight: 195 g (with tip). |
| Precision | Accuracy: ≤ 20 μm; Resolution: 15 μm; Scanning speed: 1,500 frames per second. Compatible with full-arch, crown, bridge, and basic orthodontic workflows. | Accuracy: ≤ 12 μm; Resolution: 8 μm; Scanning speed: 3,000 frames per second. Enhanced depth perception and motion tolerance. Optimized for complex implantology, digital smile design, and high-accuracy orthodontic staging. |
| Material | Medical-grade polycarbonate housing with silicone grip. Scanner tip: autoclavable zirconia-ceramic composite (up to 134°C, 20 cycles). | Antimicrobial nano-coated magnesium alloy chassis with textured medical silicone grip. Scanner tip: sapphire-reinforced ceramic with hydrophobic coating; autoclavable up to 135°C, 50 cycles. IP54 rated for dust and splash resistance. |
| Certification | CE Mark (MDD 93/42/EEC), FDA 510(k) cleared (Class II), ISO 13485:2016 compliant, RoHS certified. | CE Mark (MDR 2017/745), FDA 510(k) cleared (Class II), Health Canada licensed, ISO 13485:2016, IEC 60601-1 (3rd Ed.), IEC 60601-1-2 (4th Ed.), GDPR-compliant data handling, HIPAA-ready. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Industry Context: The global intraoral scanner market is projected to reach $3.2B by 2026 (CAGR 11.2%). While “Itero” is a registered trademark of Align Technology, this guide addresses sourcing clinically equivalent, CE/ISO-certified intraoral scanners from Chinese OEM/ODM manufacturers. Caution: Legitimate suppliers will NOT market devices as “Itero clones” due to IP infringement risks. Focus on technical specifications and regulatory compliance.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Clinical Deployment)
Chinese manufacturers often display certification badges without valid documentation. Follow this verification protocol:
| Critical Verification Point | Validation Method | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Certification (Quality Management System) |
Request certificate # via email. Cross-verify with iso.org or issuing body (e.g., TÜV, SGS). Confirm scope includes “design and manufacture of intraoral scanners”. | Clinical liability exposure; device rejection by customs (EU/US); voided warranties. |
| CE Marking (MDR 2017/745) | Demand full EU Declaration of Conformity with Article 10 MDR references. Verify notified body number (e.g., 0123) via NANDO database. | Illegal to sell in EEA; potential €20k+ fines per device; clinic malpractice claims. |
| EMC & Electrical Safety (IEC 60601-1, IEC 60601-1-2) |
Request test reports from accredited lab (e.g., CETL, TÜV Rheinland). Confirm tests cover scanner + charging station. | Device malfunction risk; fire hazards; insurance policy invalidation. |
Why Shanghai Carejoy Excels in Compliance (Case Study)
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains active:
• ISO 13485:2016 Certificate # CQM1841678 (Valid until 03/2027)
• CE MDR Certificate # DE/0123/1841678 (Issued by TÜV SÜD)
• Full technical documentation available for audit upon NDA. All scanners undergo 72-hour burn-in testing pre-shipment.
Step 2: Negotiating MOQ & Commercial Terms
Chinese suppliers use MOQs to filter serious buyers. Strategic negotiation framework:
| Buyer Type | Typical MOQ Range | 2026 Negotiation Strategy | Key Leverage Point |
|---|---|---|---|
| Dental Clinics (Direct Purchase) |
1-5 units | Accept sample unit at 150% price. Negotiate MOQ waiver for 3+ units with annual service contract. | Long-term service revenue potential (scanners require calibration/software updates). |
| Distributors (Regional/Exclusive) |
10-50 units | Link MOQ to territory size: e.g., 20 units for country-wide distribution. Demand consignment stock option for slow-moving SKUs. | Market penetration commitment (require 6-month sales forecast). |
| OEM Partners (White-label) |
50+ units | Negotiate tiered pricing: 45 units @ $X, 55 units @ $Y. Insist on free firmware customization for first 100 units. | IP ownership of custom UI elements and branding. |
Step 3: Shipping & Logistics (DDP vs. FOB)
2026 tariff structures and Incoterms significantly impact landed costs:
| Term | Cost Responsibility | 2026 Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer pays freight, insurance, customs clearance. Supplier handles port fees. | Customs delays (avg. 7-14 days in EU 2026); unexpected port surcharges; inventory in transit risk. | Experienced distributors with in-house logistics teams. |
| DDP (Delivered Duty Paid) | Supplier quotes all-inclusive price to clinic/distributor door. | Supplier may inflate costs; limited visibility into customs process; force majeure risks. | 90% of clinics and new-market distributors (simplifies budgeting). |
Shanghai Carejoy’s Logistics Advantage
Leveraging 19 years of export experience from Baoshan District (Shanghai Port Zone):
• DDP Solutions: Fixed landed cost quotes to 35+ countries (includes 2026 EU battery import fees)
• FOB Support: Pre-shipment documentation audit to prevent customs holds
• Transit Time: 18-22 days door-to-door (EU/US via air freight; 30 days for sea)
Strategic Partner Recommendation: Shanghai Carejoy Medical
As a vertically integrated manufacturer (not a trading company), Carejoy eliminates middleman markups while ensuring compliance:
- Core Strength: In-house R&D team (27 engineers) with 5 scanner patents filed in 2025
- Quality Control: 100% end-of-line testing with traceable calibration certificates
- After-Sales: 24-month warranty; EU-based service centers in Germany & Poland
Next Steps for Verified Sourcing
Contact Shanghai Carejoy for 2026 Procurement:
📧 [email protected] | 💬 WhatsApp: +86 159 5127 6160
📍 Factory: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Request: “2026 Dental Scanner Sourcing Kit” (Includes ISO certificates, DDP calculator, and MOQ matrix)
Disclaimer: This guide reflects 2026 regulatory landscapes. “Itero” is a trademark of Align Technology, Inc. Shanghai Carejoy Medical Co., LTD manufactures ISO-certified intraoral scanners under its own brand and OEM programs. Always conduct independent due diligence. © 2026 Dental Equipment Consultants Association. Not for public distribution.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs for Purchasing the iTero Dental Scanner in 2026
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the iTero Element™ 5D Plus scanner support for international installations in 2026? | The iTero Element 5D Plus operates on a universal input voltage range of 100–240V AC, 50/60 Hz, making it compatible with global power standards. An auto-switching power supply ensures seamless integration in clinics across North America, Europe, Asia, and other regions without requiring external transformers. Always verify local regulatory compliance (e.g., CE, FDA, Health Canada) and use manufacturer-approved power cords for your region. |
| 2. Are spare parts for the iTero scanner readily available, and how does Align Technology support long-term serviceability? | Yes, all critical spare components—including scan heads, handpiece cables, calibration tools, and charging docks—are available through authorized Align Technology distributors and service partners. As of 2026, Align maintains a global spare parts logistics network with regional distribution centers ensuring 3–5 business day delivery for standard components. Registered clinics gain access to the iTero Service Portal for real-time part tracking, predictive maintenance alerts, and firmware updates to extend equipment lifecycle. |
| 3. What does the installation process involve, and is on-site technician support included? | Installation of the iTero scanner includes hardware setup, software integration with clinic management systems (e.g., exocad, 3Shape, or intraoral workflow platforms), and network configuration. As of 2026, all new iTero purchases include complimentary on-site installation by an Align-certified technician within 10 business days of delivery. The technician performs system calibration, validates image accuracy, and provides hands-on training for clinical and administrative staff to ensure rapid operational readiness. |
| 4. What warranty coverage is provided with the iTero scanner, and are extended service plans available? | The iTero Element 5D Plus comes with a standard 3-year comprehensive warranty covering parts, labor, and scan head performance degradation. This includes preventive maintenance visits every 12 months. Extended warranty options are available up to 5 years, with premium tiers offering 24/7 technical support, next-business-day on-site repair, and free firmware upgrades. Distributors may bundle service contracts tailored to clinic volume and geographic service accessibility. |
| 5. How does Align Technology ensure compatibility with evolving clinic IT infrastructure and data security standards in 2026? | The 2026 iTero platform supports HIPAA- and GDPR-compliant data handling with end-to-end encryption, local or cloud-based storage (via Align Secure Cloud), and integration with HL7 and DICOM standards. The system is designed for compatibility with modern clinic networks, supporting Wi-Fi 6, gigabit Ethernet, and two-factor authentication. Regular security patches and OS updates are delivered remotely, ensuring compliance with evolving cybersecurity regulations in dental healthcare environments. |
Note: Specifications and service offerings are subject to change. Always consult the latest technical documentation and service agreements from Align Technology or authorized distributors prior to procurement.
Need a Quote for Itero Dental Scan?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


