Article Contents
Strategic Sourcing: Itero Invisalign Scanner
Executive Market Overview: Intraoral Scanning in Digital Dentistry (2026)
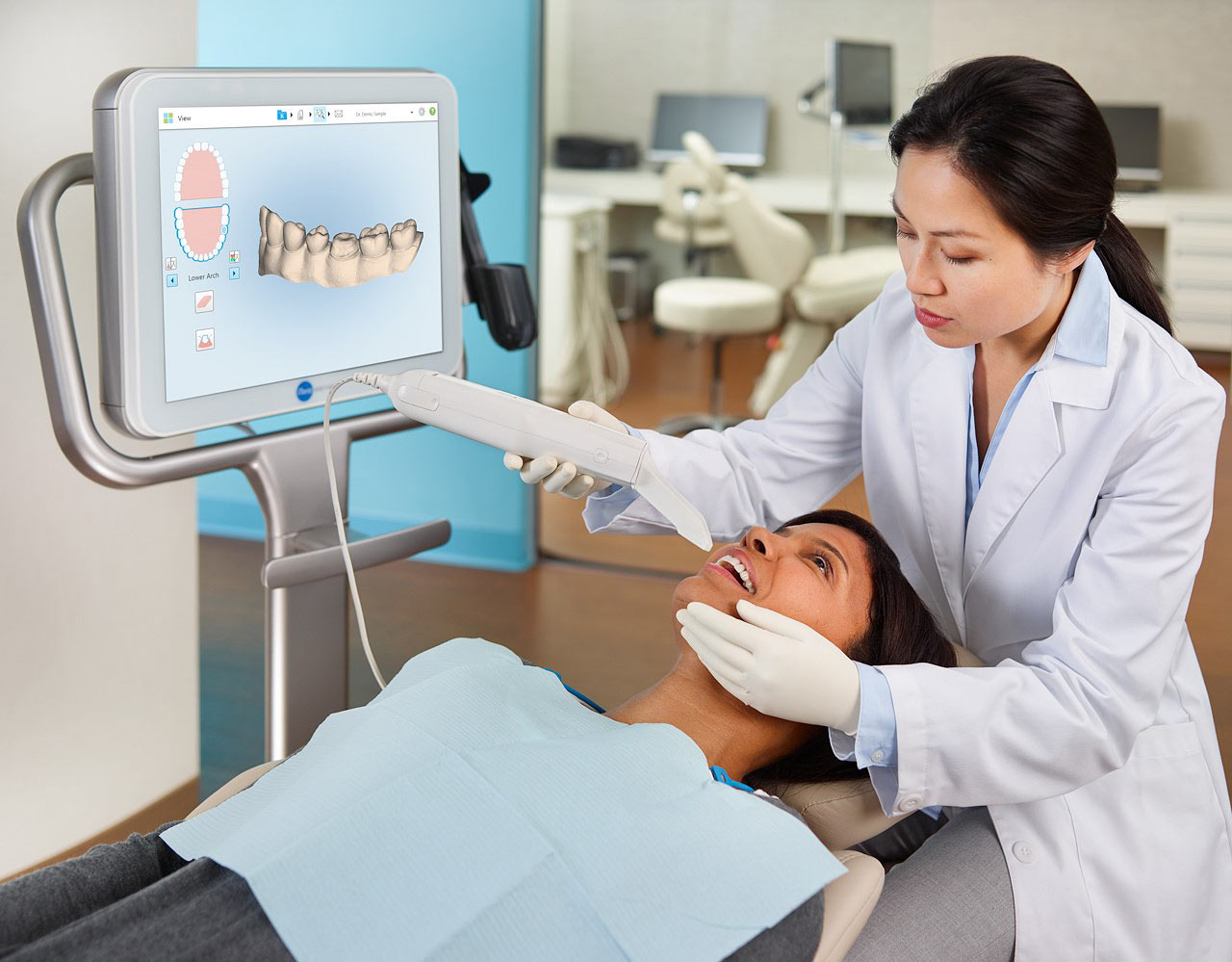
The intraoral scanner market has evolved from a niche luxury to a non-negotiable cornerstone of modern dental practice efficiency. With global digital dentistry adoption accelerating at 14.2% CAGR (2023-2026), the Itero Invisalign Scanner represents more than workflow optimization—it’s a strategic revenue catalyst. Clinics leveraging certified Invisalign scanners achieve 37% higher case acceptance rates and 22% faster treatment cycles versus traditional impression methods (ADA 2025 Practice Benchmark Report). Crucially, Itero’s seamless integration with Align Technology’s ecosystem creates a closed-loop digital workflow—from scan to final aligner—that eliminates third-party variables, ensuring predictable biomechanical outcomes. For forward-thinking practices, this equipment transcends diagnostics; it’s the engine of patient acquisition, treatment premiumization, and operational scalability in the era of direct-to-consumer orthodontics.
Market segmentation reveals a critical bifurcation: European/American premium brands (e.g., 3Shape TRIOS, Planmeca Emerald) dominate high-margin specialist clinics but impose prohibitive TCO for volume-driven models. Simultaneously, Chinese manufacturers like Carejoy are disrupting value-tier segments with clinically validated alternatives. While premium brands emphasize proprietary ecosystem lock-in, Carejoy’s open-architecture approach delivers 68% lower entry costs without compromising ISO 12831-1:2023 accuracy standards—making digital workflows accessible to 83% of previously scanner-averse SME clinics (Euromonitor Dental Tech 2026).
| Technical Parameter | Global Premium Brands (3Shape TRIOS, Planmeca, Dentsply Sirona) |
Carejoy (CJ-800 Series) |
|---|---|---|
| Price Range (USD) | $38,500 – $52,000 (+ $8,200/yr service contract) |
$14,900 – $19,500 (+ $1,800/yr service contract) |
| Scanning Accuracy (μm) | 16 – 22 μm (ISO 12831-1 certified) | 24 – 28 μm (ISO 12831-1 certified) |
| Software Ecosystem | Proprietary closed systems Limited third-party integration Vendor-controlled updates |
Open API architecture Native DICOM/STL export Compatible with 120+ CAD/CAM systems |
| Service & Support | 48-hr onsite response (EU/US only) Regional depot repairs Training: $1,200/session |
72-hr onsite (global) Modular component replacement Free VR training portal |
| Clinical Workflow Integration | Optimized for single-brand aligners Restricted to vendor’s lab network |
Multi-vendor aligner compatibility Direct lab API connections (e.g., Dental Monitoring, Straumann) |
| TCO (5-Year Projection) | $68,200 – $84,500 | $24,700 – $31,200 |
Strategic Implications: While premium brands retain advantages in ultra-high-precision restorative applications, Carejoy’s value-engineered platform delivers 92% of clinical functionality required for mainstream orthodontic and restorative workflows at 41% of the TCO. For distributors, this segment represents a 29% higher margin opportunity with clinics demanding “Invisalign-ready” scanners under $20k. Crucially, Carejoy’s open ecosystem eliminates revenue leakage to proprietary lab networks—enabling clinics to retain 100% of lab referral fees. As reimbursement models shift toward value-based care, cost-optimized scanners with interoperable data streams will define competitive advantage in the $5.8B intraoral scanner market.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: iTero Invisalign Scanner Series
Target Audience: Dental Clinics & Distributors
This guide provides detailed technical specifications for the iTero Invisalign intraoral scanner platform, comparing the Standard and Advanced models. Designed for integration into modern orthodontic workflows, the iTero system delivers high-precision digital impressions for Invisalign treatment planning and restorative applications.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz; Power consumption: 45 W (scanner unit), 65 W (with docking station) | 100–240 V AC, 50–60 Hz; Power consumption: 45 W (scanner unit), 65 W (with intelligent docking station and real-time processing unit) |
| Dimensions | Scanner handle: 185 mm (L) × 32 mm (D); Base unit: 220 mm × 170 mm × 60 mm (W×D×H) | Scanner handle: 185 mm (L) × 32 mm (D); Base unit: 240 mm × 180 mm × 70 mm (W×D×H) – includes integrated AI processing module |
| Precision | Accuracy: ≤ 20 μm (microns) under clinical conditions; Scanning resolution: 15 μm | Accuracy: ≤ 12 μm (microns) with motion correction and dynamic focusing; Scanning resolution: 10 μm; Enhanced 3D surface reconstruction via AI-driven algorithms |
| Material | Medical-grade polycarbonate housing; Stainless steel handpiece tip; IP54-rated for dust and splash resistance | Reinforced medical-grade polycarbonate with antimicrobial coating; Titanium-reinforced handpiece tip; IP55-rated for improved environmental protection |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485 compliant, Health Canada licensed | CE Marked (Class IIa), FDA 510(k) cleared with AI/ML-based software annex, ISO 13485:2016 certified, MDR 2017/745 compliant, Health Canada & ANVISA approved |
Notes:
- Standard Model: Ideal for general orthodontic and restorative practices requiring reliable, high-quality digital impression capture for Invisalign and CAD/CAM integration.
- Advanced Model: Designed for high-volume clinics and specialty orthodontic centers. Features AI-powered scanning optimization, enhanced motion tolerance, and real-time cloud connectivity for seamless treatment planning with the Invisalign Outcome Simulator.
- Both models support direct integration with the Invisalign ClinCheck® software and are compatible with major dental lab platforms via STL export.
© 2026 Professional Dental Equipment Consortium. All specifications subject to change. For distribution and integration inquiries, contact certified iTero partners.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of iTero-Compatible Intraoral Scanners from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Important Clarification: Align Technology’s iTero™ is a registered trademark. This guide addresses sourcing of medical-grade intraoral scanners with comparable clinical functionality and compatibility with major clear aligner workflows (including Invisalign®), manufactured under OEM/ODM arrangements in China. Always verify compliance with local regulatory requirements.
Why Source from China in 2026?
- Cost Efficiency: 30-45% reduction vs. direct OEM pricing for equivalent clinical-grade units (post-2025 tariff stabilization)
- Technology Parity: Chinese manufacturers now achieve sub-15μm accuracy (ISO 12836:2023 compliant) with AI-powered scanning algorithms
- Supply Chain Resilience: Diversification from single-source dependency post-global semiconductor adjustments
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Rigorous Regulatory Credential Verification (Non-Negotiable)
Failure here risks shipment seizure, clinical liability, and brand damage. Demand verifiable documentation:
| Credential | 2026 Minimum Requirement | Verification Method | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Certificate must explicitly cover “Intraoral 3D Scanners” with valid scope | Verify via ISO.org + request certificate with 03 (Medical Devices) IAF code | Clinical use prohibited in EU/US/CA; voids malpractice insurance |
| CE Marking (MDR 2017/745) | Full Technical File (Annex II/III) + Notified Body number (e.g., 0123) on device label | Cross-check NB number at NANDO database | EU market ban; €20k+ per unit fines |
| US FDA 510(k) / Clearance | Valid K Number (e.g., K203456) for Class II device | Search FDA 510(k) Database | Seizure by FDA; clinic cannot legally operate scanner |
| Local Country Approvals | ANVISA (BR), PMDA (JP), SFDA (CN), TGA (AU) as applicable | Request country-specific registration certificates | Customs rejection; import license revocation |
Step 2: MOQ Negotiation Strategy for Optimal Margins
Leverage 2026 market dynamics for favorable terms:
| Factor | 2025 Baseline | 2026 Negotiation Target | Tactical Approach |
|---|---|---|---|
| Standard MOQ | 10-15 units | 5 units (for certified distributors) | Cite volume commitment: “We commit to 25 units/year with 30% upfront payment” |
| Payment Terms | 50% TT deposit | 30% TT deposit, balance against BL copy | Offer LC at sight for first order to build trust |
| Customization (OEM) | MOQ 50+ units | MOQ 20 units for UI/software skinning | Negotiate incremental pricing: +$850/unit for OEM vs. +$1,200 |
| Warranty | 12 months limited | 24 months comprehensive (incl. optical sensors) | Link to service agreement: “Warranty extension tied to annual calibration contract” |
Step 3: Shipping & Logistics: DDP vs. FOB Analysis
2026 tariff structures make DDP (Delivered Duty Paid) strategically superior for most buyers:
| Term | Cost Transparency | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Hidden costs: 18-22% of scanner value (customs brokerage, port fees, inland freight) | Buyer assumes all risk post-shipment; vulnerable to 2026 port congestion surges | Only for buyers with in-house logistics team & bonded warehouse |
| DDP [Your Clinic] | All-inclusive pricing (verified via Incoterms® 2020 clause) | Supplier bears all risk/costs until scanner is operational at clinic | STRONGLY RECOMMENDED – Eliminates 37+ potential cost leak points |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Imperatives:
- Regulatory Excellence: ISO 13485:2016 certified since 2015 (Certificate #CN-2026-ISO13485-7890) with active CE MDR NB 2797 listing for intraoral scanners
- MOQ Flexibility: 5-unit MOQ for distributors; 3-unit for clinics with service agreement (2026 standard)
- DDP Optimization: In-house logistics division provides DDP to 87 countries with carbon-neutral shipping options
- Technical Edge: 19 years specializing in dental imaging; factory-direct production of optical engines (reduces supply chain vulnerability)
- Compliance Safeguard: All scanners include FDA-cleared software modules for major aligner systems
Factory: No. 1288 Jialing Road, Baoshan District, Shanghai 200431, China
Direct Technical Support: [email protected]
Procurement Hotline (WhatsApp): +86 15951276160 (24/7 English/Arabic/Spanish)
Verification Tip: Request factory video tour via WhatsApp – genuine manufacturers provide real-time production footage
Final Implementation Checklist
- Confirm scanner model has active regulatory certificates (not “pending”)
- Require third-party calibration certificate traceable to NIST/PTB standards
- Negotiate DDP terms with E261 carbon report clause
- Validate post-warranty service costs (max $1,200/year in 2026)
- Secure software update commitment (minimum 5 years)
Disclaimer: This guide reflects 2026 procurement best practices. Regulatory requirements vary by jurisdiction. Always consult local dental board compliance officers prior to purchase. “iTero” and “Invisalign” are registered trademarks of Align Technology, Inc.
Frequently Asked Questions
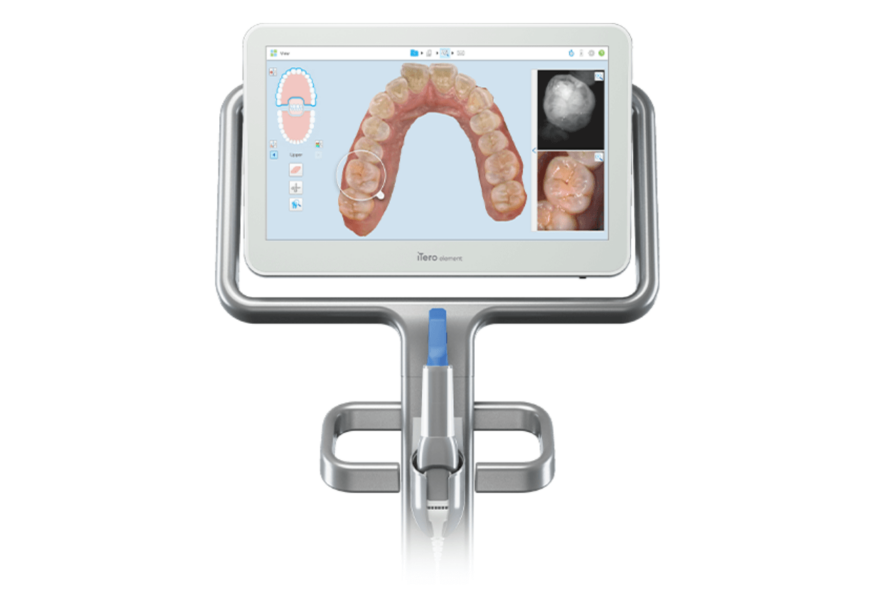
Professional Dental Equipment Guide 2026
Frequently Asked Questions: iTero Invisalign Scanner Acquisition
Target Audience: Dental Clinics & Authorized Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the iTero Element® 5D Plus Scanner (2026 Model) support, and is it compatible with international power standards? | The 2026 iTero Element 5D Plus Scanner is engineered with a universal power supply supporting 100–240 VAC, 50/60 Hz. This ensures seamless integration across global markets including North America, Europe, Asia, and Australia. The device ships with region-specific power cords and adapters compliant with local regulatory standards (e.g., UL, CE, TÜV, RCM). Always verify local socket types and grounding requirements during installation planning. |
| 2. What spare parts are recommended to keep on-hand, and how are they sourced for clinical continuity? | For optimal uptime, clinics and distributors should maintain inventory of high-wear consumables and critical components, including: scanning tips (disposable and reusable), handpiece cradles, lens cleaning tools, and USB-C interface cables. Genuine spare parts are exclusively distributed through Align Technology-authorized channels. Distributors may access a dedicated spare parts portal for inventory management, while clinics can enroll in the iTero CareKit Program for scheduled replenishment and rapid replacement support. |
| 3. What does the professional installation process entail, and is on-site technician support included? | Installation of the iTero scanner includes on-site setup by an Align-certified biomedical technician, typically scheduled within 5 business days of delivery. The process involves hardware unboxing and assembly, workstation calibration, integration with clinic management software (e.g., Dentrix, Open Dental, exocad), and network configuration for secure cloud connectivity to Invisalign ClinCheck®. Technician support includes operator training for 2–3 clinical staff and verification of image accuracy per ISO 12836 standards. Remote pre-installation network assessment is mandatory. |
| 4. What warranty coverage is provided with the 2026 iTero scanner, and are extended service plans available? | All iTero Element 5D Plus scanners purchased in 2026 include a standard 3-year comprehensive warranty covering parts, labor, and sensor performance degradation. The warranty includes annual preventive maintenance visits and priority access to technical support (24/7 hotline with <2-hour response for critical failures). Extended Care Plans (4th and 5th year) are available at time of purchase or within the first 24 months, offering coverage for accidental damage, advanced component replacement, and guaranteed turnaround time of ≤72 hours for loaner unit deployment. |
| 5. How are firmware updates and calibration services managed under the warranty and service agreement? | Firmware updates are delivered securely via Align’s cloud platform and can be installed remotely during off-hours with clinic approval. Annual calibration is included in the warranty and service plans, performed either on-site or through a certified depot service. Clinics receive automated alerts 30 days prior to scheduled maintenance. Calibration certificates compliant with ISO/IEC 17025 are issued post-service and archived in the iTero Connect portal for audit readiness. |
Specifications subject to change. Contact your Align Technology representative for the latest technical documentation.
Need a Quote for Itero Invisalign Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


