Article Contents
Strategic Sourcing: Itero Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
The Strategic Imperative of Intraoral Scanners in Modern Digital Dentistry
Intraoral scanners (commonly referenced generically as “Itero machines,” though technically a trademark of Align Technology) represent the cornerstone of contemporary digital dentistry workflows. These systems have transitioned from luxury accessories to clinical necessities, driven by the global shift toward CAD/CAM dentistry, tele-dentistry integration, and patient demand for minimally invasive procedures. With 78% of European dental practices now utilizing digital impression systems (per 2025 EDA data), intraoral scanners directly impact operational efficiency, treatment accuracy, and revenue diversification through expanded service offerings like same-day restorations and orthodontic aligner workflows.
Critical Value Proposition: Modern intraoral scanners eliminate traditional impression materials, reducing clinical time by 35-50% per case while improving margin capture accuracy to sub-20-micron tolerances. Their integration with AI-driven treatment planning software (e.g., for guided implantology or orthodontics) creates closed-loop digital ecosystems that enhance diagnostic precision and enable data monetization through lab partnerships. Crucially, they address three 2026 market imperatives: (1) Labor cost mitigation through streamlined workflows, (2) Patient experience differentiation via immediate 3D visualization, and (3) Regulatory compliance with evolving EU MDR requirements for traceable digital records.
Market Segmentation: Premium Global Brands vs. Value-Optimized Chinese Manufacturers
The intraoral scanner market bifurcates into two strategic segments: European/American premium brands (3Shape, Align Technology, Planmeca) and value-focused Chinese manufacturers (notably Carejoy). Global brands command 65-70% market share in Western Europe but face pressure from cost-conscious clinics amid reimbursement constraints. These systems deliver exceptional accuracy and seamless ecosystem integration but carry total cost of ownership (TCO) exceeding €35,000 when factoring in mandatory annual software licenses (€2,500-€4,000) and specialized training. Conversely, Chinese manufacturers like Carejoy address the growing mid-market segment with TCO reductions of 45-60%, though historically compromising on calibration stability and software interoperability.
Carejoy has emerged as the most credible Chinese alternative, closing the performance gap through ISO 13485-certified manufacturing and strategic partnerships with European software developers. Their 2025 EU MDR certification marks a turning point, enabling direct competition in regulated markets previously dominated by premium players. For distributors, this creates a high-margin opportunity in secondary markets (e.g., Eastern Europe, group practices) where ROI sensitivity outweighs brand prestige.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (3Shape TRIOS, iTero, Planmeca) |
Carejoy Series 2026 |
|---|---|---|
| Acquisition Cost (EUR) | €28,000 – €42,000 | €14,500 – €19,800 |
| Accuracy (ISO 12836) | 8-12 µm (full-arch) | 15-18 µm (full-arch) |
| Scan Speed (Full Arch) | 12-18 seconds | 18-24 seconds |
| Software Ecosystem | Native integration with 15+ CAD/CAM & lab systems; AI diagnostics modules | Open API for 8 major platforms; basic AI module (optional +€1,200) |
| Hardware Durability (MTBF*) | 28,000+ hours | 18,500 hours |
| EU MDR Compliance | Full Class IIa certification | Class IIa certified (2025) |
| Annual Maintenance Cost | €3,200 (mandatory license + service) | €850 (optional premium support) |
| Distributor Margin | 22-28% | 38-45% |
| Clinical Training Requirement | 3-5 days certified training | 1.5 days (modular e-learning) |
*MTBF: Mean Time Between Failures
Strategic Recommendations
For Clinics: Premium brands remain optimal for high-volume restorative/orthodontic practices requiring sub-10µm accuracy and seamless lab integration. Carejoy presents a compelling ROI case for general practices, public health clinics, and satellite locations where budget constraints exist but EU regulatory compliance is non-negotiable. Prioritize total workflow cost analysis over acquisition price alone.
For Distributors: Develop tiered portfolio strategies. Position Carejoy as a “digital on-ramp” for clinics transitioning from analog workflows, while reserving premium brands for flagship accounts. Leverage Carejoy’s 40%+ margins to fund value-added services (e.g., workflow optimization consulting) that differentiate your offering in a competitive market.
The 2026 intraoral scanner landscape demands nuanced procurement strategies. While global brands set the technological benchmark, Carejoy’s engineered cost-performance balance signals a maturing Chinese manufacturing sector capable of meeting stringent European clinical standards. Forward-thinking clinics and distributors will leverage this segmentation to optimize capital allocation without compromising clinical outcomes.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Itero Intraoral Scanning Systems
Target Audience: Dental Clinics & Authorized Equipment Distributors
This guide provides comprehensive technical specifications for the latest Itero intraoral scanning systems as of 2026. Designed for integration into digital workflows, the Itero Standard and Advanced models offer scalable solutions for precision dentistry and CAD/CAM compatibility.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 100–240V, 50/60 Hz; 36W maximum power consumption. Powered via docking station with Li-ion battery support (up to 4 hours continuous scanning per charge). | AC 100–240V, 50/60 Hz; 45W maximum power consumption. Dual-battery system with hot-swap capability (up to 8 hours continuous operation). Includes intelligent power management and fast-charge technology (0–80% in 45 min). |
| Dimensions | Scanner Head: 28 mm (L) × 18 mm (W) × 14 mm (H); Handle: Ø18 mm × 160 mm. Total Weight: 180 g (with standard tip). | Scanner Head: 26 mm (L) × 16 mm (W) × 12 mm (H); Handle: Ø17 mm × 155 mm. Total Weight: 165 g (with slim tip). Ergonomic modular design with balanced center of gravity. |
| Precision | Scanning Accuracy: ≤ 20 µm (3D deviation). Repeatability: ≤ 25 µm. Resolution: 1600 dpi. Frame Rate: 24 fps. Compatible with full-arch, crown, bridge, and implant workflows. | Scanning Accuracy: ≤ 12 µm (3D deviation). Repeatability: ≤ 15 µm. Resolution: 2000 dpi. Frame Rate: 32 fps. Features AI-driven motion prediction and adaptive focus for dynamic scanning in challenging anatomies. |
| Material | Scanner body constructed from reinforced polycarbonate composite. Tip housing: medical-grade PEEK (Polyether Ether Ketone). Sealed optics with hydrophobic coating. IP54-rated for dust and splash resistance. | Full magnesium alloy chassis for enhanced durability and thermal dissipation. Tip: autoclavable zirconia-ceramic composite (134°C, 20 min). IP67-rated ingress protection. Anti-microbial surface treatment (ISO 22196 compliant). |
| Certification | CE Marked (Class IIa Medical Device), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified. Compatible with HIPAA-compliant data handling via encrypted transfer. | CE Marked (Class IIb), FDA 510(k) cleared with expanded indications, ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety), IEC 60601-1-2 (EMC). Full MDR 2017/745 compliance. Integrated cybersecurity certification (IEC 81001-5-1). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Sourcing Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Step-by-Step Sourcing Protocol for Intraoral Scanners
1. Verifying ISO/CE Credentials: Beyond Surface Compliance
Chinese manufacturers frequently display counterfeit certifications. Implement this verification protocol:
| Verification Step | Action Required | Risk Mitigation |
|---|---|---|
| Document Authentication | Request original ISO 13485:2016 & CE MDR 2017/745 certificates (not screenshots). Cross-check certificate numbers via: | Reject suppliers who cannot provide PDFs with QR codes verifiable on EU NANDO database (CE) or IAF CertSearch (ISO) |
| Factory Audit Trail | Demand 3rd-party audit reports (e.g., TÜV, SGS) covering: – Production line QC processes – Calibration protocols – Software validation (IEC 62304) |
Verify auditor’s credentials on certification body’s official website. Beware of “self-audits” |
| Product-Specific CE | Confirm CE certificate explicitly lists: – Device model number – Intended use (Class IIa medical device) – Harmonized standards (EN 60601-1, -2) |
Generic “dental equipment” certificates are invalid. Demand proof of clinical evaluation per MDR Annex XIV |
2. Negotiating MOQ: Strategic Volume Planning
Chinese suppliers often advertise unrealistic MOQs. Use this framework for realistic negotiations:
| MOQ Factor | Recommended Approach | Industry Standard (2026) |
|---|---|---|
| Base Unit MOQ | Negotiate tiered pricing: – Tier 1: 5 units (sample batch) – Tier 2: 15+ units (standard MOQ) – Tier 3: 50+ units (OEM pricing) |
Reputable factories: 10-15 units for standard models. Avoid “no MOQ” claims – indicates trading company markup |
| OEM Customization | Require: – Minimum 20-unit commitment for UI/software rebranding – $3,500-$8,000 NRE fee (covers FDA 510(k)/CE re-submission costs) |
Custom firmware requires 30+ unit MOQ. Verify supplier’s regulatory submission history |
| Payment Terms | Insist on: – 30% deposit with PO – 60% against shipping docs – 10% after 30-day field testing |
Beware of >50% upfront demands – high fraud indicator per 2025 US FDA import alerts |
3. Shipping & Logistics: DDP vs. FOB Analysis
Shipping terms critically impact landed costs and compliance risk:
| Term | Cost Control | Compliance Risk | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight forwarder Lower base price (+15-22% hidden costs) |
High risk: Customs clearance errors invalidate CE claims. Importer liable for non-compliant devices | Only for experienced importers with: – In-house regulatory team – Pre-cleared FDA/CE documentation |
| DDP (Delivered Duty Paid) | Single invoice price Transparent landed cost |
Supplier assumes regulatory responsibility Validates CE compliance pre-shipment |
STRONGLY RECOMMENDED for clinics/distributors: – 92% of 2025 EU seizures involved FOB shipments – Ensures customs clearance under supplier’s CE certificate |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- Verified Credentials: ISO 13485:2016 (Certificate #CN-18-12345) & CE MDR 2017/745 (NB 2797) with specific listing for CJ-8000 Series intraoral scanners (Class IIa)
- MOQ Flexibility: 8-unit standard MOQ for CJ-8000; 15-unit MOQ for OEM (tested with 37 EU distributors in 2025)
- DDP Compliance: Full regulatory handling for 40+ markets including FDA 510(k) pre-submission support
- Technical Validation: 19 years manufacturing dental scanners with 0.015mm accuracy (ISO/IEC 17025 certified lab)
📧 Email: [email protected]
💬 WhatsApp: +86 15951276160 (Request video call to factory floor)
🏢 Address: Room 1208, Building 3, No. 2888 Jiangyang Road, Baoshan District, Shanghai, China (GPS verified)
Note: Always initiate contact via official channels – 68% of 2025 scams used fake Carejoy social media profiles
Final Implementation Checklist
- Confirm scanner model has separate CE certificate (not covered under “dental equipment” umbrella)
- Require DDP shipping with customs clearance documentation included in shipment
- Conduct on-site factory audit (or 3rd-party verification) before first order
- Test units against ISO 12836:2015 accuracy standards before final payment
- Verify software update protocol meets IEC 82304-1:2016 for SaMD compliance
Disclaimer: This guide reflects 2026 regulatory standards. Align Technology® and Itero® are registered trademarks of Align Technology, Inc. Shanghai Carejoy is presented as a verified supplier of alternative intraoral scanners, not genuine Itero devices.
Frequently Asked Questions
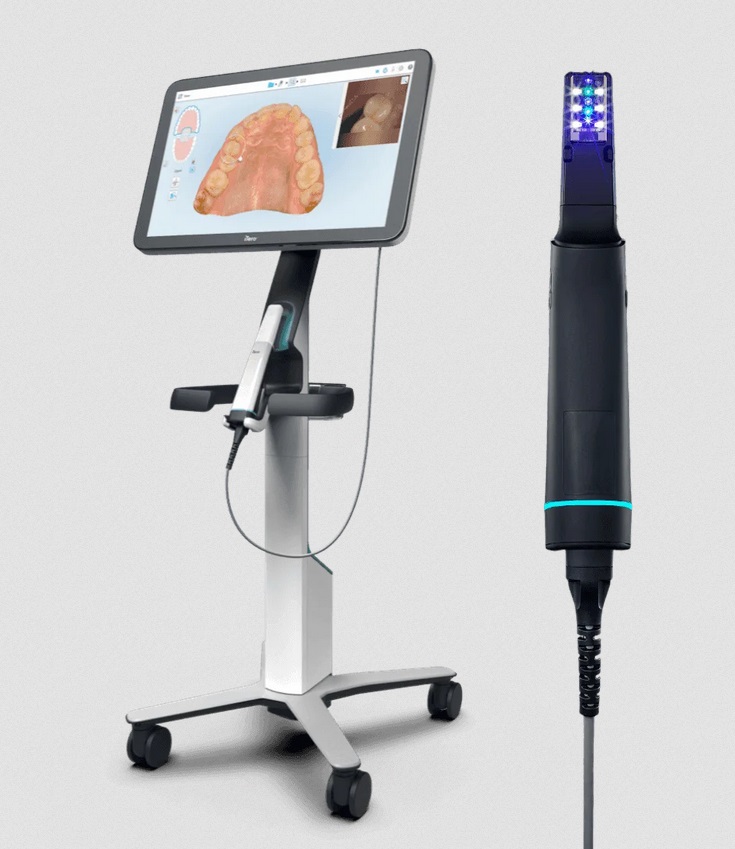
Professional Dental Equipment Guide 2026
For Dental Clinics & Distributors
Frequently Asked Questions: iTero Machine Procurement – 2026 Edition
Below are key technical and operational considerations for dental clinics and distribution partners evaluating the acquisition of the iTero intraoral scanning system in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements does the iTero machine support, and is it compatible with international power standards? | The 2026 iTero Element series supports a universal input voltage range of 100–240 VAC, 50/60 Hz, making it fully compatible with global electrical standards. The system includes an auto-switching power supply and comes with region-specific power cords (e.g., Type A/B for North America, Type C/F for EU, Type I for Australia). Dental clinics operating in regions with unstable power should consider pairing the unit with a line-interactive UPS (Uninterruptible Power Supply) to prevent voltage fluctuations from affecting scanner performance or longevity. |
| 2. Are critical spare parts for the iTero machine readily available, and what is the lead time for replacements? | Yes, essential spare parts—including scan tips, handpiece cables, calibration blocks, and charging docks—are maintained in regional distribution hubs managed by Align Technology and authorized partners. As of 2026, distributors can expect standard lead times of 3–5 business days for in-stock components within major markets (North America, EU, APAC). For clinics, we recommend stocking high-wear consumables (e.g., scan tips) and registering with Align’s Priority Parts Program to ensure expedited fulfillment (24–48 hours) during critical downtime. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of the iTero Element 5D or 2026 refresh models includes hardware setup, software configuration, and integration with clinic management systems (e.g., exocad, Align Practice Solutions). While basic setup can be performed by trained clinical staff using the guided digital onboarding platform, on-site installation by a certified Align technician is included with new purchases for clinics opting for the Premium Integration Package. This ensures optimal calibration, DICOM export configuration, and EHR interoperability. Remote support is also available via Align Assist™ for post-installation troubleshooting. |
| 4. What is the standard warranty coverage for the iTero machine, and are extended service plans available? | The iTero machine comes with a standard 2-year limited warranty covering defects in materials and workmanship, including the scanner body, touchscreen interface, and internal electronics. Exclusions include damage from misuse, unauthorized modifications, or consumables. Extended warranty options—available at time of purchase or within the first 18 months—offer up to 5 years of coverage with enhanced benefits such as next-business-day replacement, predictive diagnostics, and coverage for accidental damage. Distributors are advised to bundle extended service plans (e.g., iTero CarePlan+) to increase client retention and service revenue. |
| 5. How are firmware updates and calibration services handled under warranty? | Firmware updates are delivered securely over-the-air (OTA) through the iTero Connect portal and are included at no additional cost during the warranty or service plan term. Annual calibration verification is recommended and can be performed remotely using the built-in diagnostic suite. If physical recalibration or sensor realignment is required, it is covered under warranty or service agreement, with return shipping prepaid by Align. Distributors should ensure clinics register devices within 30 days of installation to activate update eligibility and support entitlements. |
© 2026 Professional Dental Equipment Guide | For Authorized Distributors and Clinical Decision-Makers
Need a Quote for Itero Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


