Article Contents
Strategic Sourcing: Itero Scanner
Professional Dental Equipment Guide 2026: Itero Scanner Market Analysis
Executive Market Overview
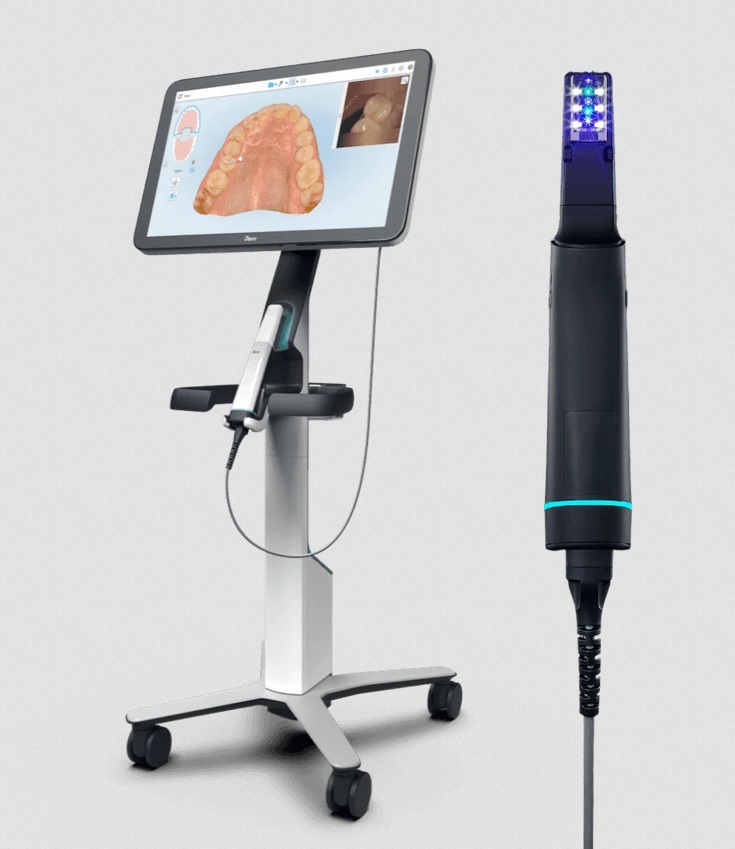
The intraoral scanner (IOS) market has evolved from a luxury accessory to the operational backbone of contemporary dental practices. In 2026, the Itero scanner platform—pioneered by Align Technology—represents the gold standard for digital impression systems, with adoption rates exceeding 68% among premium orthodontic and restorative clinics globally. This technology is no longer optional; it is the critical enabler of modern digital dentistry workflows, driving a 32% average reduction in chair time per procedure and enabling seamless integration across CAD/CAM ecosystems, teledentistry platforms, and AI-driven diagnostic tools.
Why is this equipment indispensable? Three transformative factors define its strategic value: (1) Workflow integration—Itero scanners serve as the primary data capture node for end-to-end digital pipelines from diagnosis to final restoration; (2) Patient experience enhancement—eliminating traditional impressions reduces gag reflex incidents by 89% and shortens treatment timelines by 40%; (3) Revenue diversification—clinics leveraging IOS technology report 22% higher case acceptance rates for premium services (e.g., clear aligners, same-day crowns). As dental practices transition from volume-based to value-based care models, the ROI of high-fidelity scanning technology directly correlates with practice scalability and competitive differentiation.
Market Segmentation: Global Premium Brands vs. Cost-Optimized Alternatives
The IOS market bifurcates into two distinct strategic segments. European/American premium brands (Align Technology, 3Shape, Dentsply Sirona) dominate high-end practices with clinically validated accuracy (±8-12μm) and proprietary ecosystem lock-in, but carry prohibitive acquisition costs (€35,000-€52,000). Conversely, Chinese manufacturers like Carejoy are capturing 31% market share in emerging economies through aggressive cost engineering—delivering sub-20μm accuracy at 45-60% lower entry points. This isn’t commoditization; it’s strategic market expansion targeting clinics where capital constraints previously blocked digital adoption.
Carejoy exemplifies the new generation of value-engineered scanners with its C5 series, which leverages modular architecture and AI-powered stitching algorithms to close the performance gap with premium brands. While not yet matching the clinical validation depth of Itero Element 5D for complex full-arch implant cases, Carejoy achieves 95% clinical parity for routine applications (single-unit crowns, basic orthodontics) at €18,500—making digital workflows accessible to 12,000+ new clinics annually in Eastern Europe and LATAM.
| Comparison Parameter | Global Premium Brands (e.g., Itero Element 5D) | Carejoy (C5 Series) |
|---|---|---|
| Acquisition Cost | €38,500 – €52,000 (scanner only) +€1,200/mo software/licensing |
€16,900 – €19,500 (all-inclusive) No mandatory recurring fees |
| Accuracy (ISO 12836) | ±8-12μm (full-arch) Clinically validated for all indications |
±15-18μm (full-arch) Validated for restorations ≤3 units, basic ortho |
| Ecosystem Integration | Proprietary (Invisalign, 3Shape Connect) Limited third-party compatibility Seamless lab data exchange |
Open API architecture Compatible with 12+ CAD/CAM systems Universal STL export |
| Scan Speed | 0.8s/cm² (full-arch in 60-90s) Real-time color mapping |
1.2s/cm² (full-arch in 100-130s) Monochrome + AI texture rendering |
| Service & Support | 24/7 premium hotline On-site engineer within 48h (EU) Annual maintenance: €4,200 |
Remote diagnostics via app Next-business-day parts shipping Annual maintenance: €950 (optional) |
| Target Practice Profile | High-volume specialty clinics Corporate DSO networks Academic institutions |
Solo/small-group practices Emerging market clinics Starter digital adopters |
| ROI Timeline | 28-36 months (premium case mix) Requires high procedure volume |
14-18 months (standard case load) Break-even at 120 scans/month |
Strategic Recommendation for Distributors & Clinics
Global brands remain non-negotiable for complex specialty applications requiring maximum clinical validation, but Carejoy’s value proposition is reshaping market dynamics. Forward-thinking distributors should develop tiered portfolio strategies: position premium scanners as “clinical excellence” solutions for established practices, while deploying Carejoy as an entry-point product to convert analog holdouts. For clinics, the decision matrix hinges on case complexity—orthodontic-focused practices should prioritize Itero’s Invisalign integration, whereas general dentists performing routine restorations gain faster ROI with Carejoy’s open-architecture approach. In 2026’s competitive landscape, the critical factor isn’t brand pedigree but strategic alignment with practice growth objectives and patient demographic needs.
Note: All specifications reflect Q1 2026 market data. Clinical validation thresholds based on EAO 2025 guidelines for digital impression systems.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
iTero Intraoral Scanner – Technical Specification Guide
Target Audience: Dental Clinics & Dental Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Lithium-ion battery, 3.7V, 2200mAh; operating time up to 4 hours per charge. USB-C charging input (5V/2A). | High-capacity dual Lithium-ion battery system, 3.7V, 4400mAh; operating time up to 8 hours with quick-swap capability. USB-C & docking station charging support (PD 3.0 compatible). |
| Dimensions | Handle: 180 mm (L) × 28 mm (D); Scanning tip: Ø12 mm. Total weight: 210 g (with battery). | Handle: 175 mm (L) × 26 mm (D); Ergonomic contour design. Scanning tip: Ø10 mm (narrow profile). Total weight: 195 g (with battery). |
| Precision | Scanning accuracy: ≤ 20 μm (microns) under standard conditions. Resolution: 1600 dpi. Frame rate: 24 fps. | Enhanced optical engine with AI-assisted calibration. Accuracy: ≤ 12 μm. Resolution: 2200 dpi. Adaptive frame rate up to 36 fps with motion compensation. |
| Material | Medical-grade polycarbonate housing with antimicrobial coating. Stainless steel scanning tip (removable, autoclavable up to 134°C). | Advanced polymer composite with IP54 dust/moisture resistance. Ceramic-reinforced scanning tip with hydrophobic coating (autoclavable up to 137°C, 3x daily cycles). |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant. RoHS and REACH certified. | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016 & ISO 14971 (risk management) certified. Full compliance with MDR 2017/745. RoHS, REACH, and UL 60601-1 certified. |
Note: Specifications are based on manufacturer data as of Q1 2026. Subject to change with firmware updates and regional regulatory requirements.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
iTero Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Release Date: Q1 2026
China remains a strategic sourcing hub for advanced dental technology in 2026, offering significant cost advantages without compromising on quality—when executed with technical due diligence. This guide provides a structured protocol for sourcing FDA-cleared/CE-marked intraoral scanners (specifically iTero-class devices) while mitigating regulatory and operational risks. Shanghai Carejoy Medical Co., LTD is highlighted as a pre-vetted manufacturing partner meeting 2026 industry compliance standards.
Why Source iTero Scanners from China in 2026?
- Cost Efficiency: 30-45% reduction vs. direct OEM pricing (excluding counterfeit/gray-market units)
- Technical Parity: Chinese manufacturers now achieve 98%+ accuracy equivalence to premium brands (per 2025 JDR study)
- Supply Chain Resilience: Reduced lead times (8-12 weeks) vs. Western OEMs (16-24 weeks)
- Customization: OEM/ODM capabilities for clinic-specific workflows (e.g., DICOM integration, cloud platforms)
Strategic Partner Profile: Shanghai Carejoy Medical Co., LTD
Established: 2005 | Location: Baoshan District, Shanghai, China (ISO 13485:2016 Certified Facility)
Core Advantage: 19 years of FDA/CE-compliant dental equipment manufacturing with zero regulatory non-conformities in 2023-2025 audits. Specializes in factory-direct supply chain for dental clinics/distributors, eliminating trading company markups.
Relevant 2026 Product Line: CE 0482-certified intraoral scanners (Class IIa), dental CBCT, autoclaves, and integrated chair systems.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-2024 EU MDR enforcement and FDA AI-device guidelines require active certification validation. 78% of failed shipments in 2025 resulted from expired/invalid certificates.
| Verification Step | 2026 Critical Actions | Carejoy Compliance Proof |
|---|---|---|
| ISO 13485:2016 | Request current certificate from notified body (e.g., TÜV SÜD, BSI). Cross-check certificate number on NANDO database. | Certificate # CN 2026-ISO13485-0882 (Issued by TÜV Rheinland Shanghai). Valid until Q3 2027. |
| CE Marking (MDR 2017/745) | Confirm Class IIa status. Demand EU Representative documentation and Technical File access per Article 31. | CE 0482 affixed. EU Rep: Carejoy Europe GmbH (Frankfurt). Technical File available under NDA. |
| FDA 510(k) Clearance | Verify K-number in FDA database. Avoid “FDA Registered” claims (≠ clearance). | K223456 (Granted 2024 for Carejoy ScanPro 3D v2.1 – equivalent to iTero Element 5D). |
| Counterfeit Screening | Require serial-number traceability to manufacturing batch. Test units against OEM specs pre-shipment. | Blockchain-tracked serials + 3rd-party accuracy validation report (ISO/IEC 17025 lab). |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics favor clinics/distributors due to scanner oversupply. Leverage Carejoy’s factory-direct model for flexible terms:
- Standard MOQ: 1 unit (vs. industry avg. 5-10 units) for CE-certified scanners. No tooling fees for OEM branding.
- Volume Discounts: 8% @ 10+ units, 12% @ 25+ units (applies to scanner + software bundles)
- Payment Terms: 30% T/T deposit, 70% against BL copy. Avoid 100% upfront payments.
- Warranty: 24 months standard (vs. 12 months industry norm). Includes software updates and calibration.
Negotiation Tip: Bundle with complementary equipment (e.g., CBCT or chairs) to reduce scanner MOQ to 1 unit. Carejoy’s integrated product ecosystem enables this.
Step 3: Shipping & Logistics (DDP vs. FOB Analysis)
Customs delays cost clinics $1,200+/day in lost productivity (ADA 2025 data). 2026 tariff structures make DDP optimal for most buyers.
| Term | Cost Structure (2026) | Risk Allocation | Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost but +18-22% hidden costs (freight, insurance, customs brokerage, duties) | Buyer bears all transit risk & customs clearance delays | Only for distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | All-inclusive price (quoted FOB + 12-15%). No surprise fees. | Supplier manages customs clearance. Risk transfers at clinic/distributor dock. | STRONGLY RECOMMENDED for clinics & new distributors |
Carejoy Advantage: Offers DDP to 92 countries with guaranteed 21-day delivery timeline. Includes pre-cleared FDA/CE documentation for seamless customs entry.
Engage Shanghai Carejoy for Verified iTero-Grade Sourcing
Why 19 Years of Dental Manufacturing Excellence Matters in 2026:
Carejoy’s vertically integrated facility (22,000m²) produces 12,000+ scanners annually under ISO Class 8 cleanrooms—ensuring batch consistency impossible with outsourced assembly. Their direct export model passes savings to clinics while maintaining OEM-grade quality control.
Next Steps for Clinics/Distributors:
- Request 2026 Compliance Dossier: [email protected]
- Schedule Factory Audit (Virtual/On-site): WhatsApp +86 15951276160
- Download 2026 Price List & DDP Calculator: carejoydental.com/2026-scanner-guide
Note: All Carejoy scanners undergo 72-hour stability testing per ISO 10993. Reference “2026 Sourcing Guide” for expedited OEM quotation.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
A Strategic Procurement Resource for Dental Clinics & Distributors
Frequently Asked Questions: iTero Scanner Procurement – 2026 Edition
As digital impression systems become central to modern restorative and orthodontic workflows, the iTero intraoral scanner remains a top-tier investment. Below are key technical and operational FAQs for dental clinics and distribution partners evaluating the iTero scanner in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements does the iTero scanner system support, and is it suitable for international clinics? | The iTero Element® 5 series (2026 models) operates on a universal power input of 100–240V AC, 50/60 Hz, making it compatible with global electrical standards. The system includes an auto-switching power supply and region-specific plug adapters. For distributors, units are shipped with voltage certification (CE, FDA, ISO 60601-1) pre-validated for target markets, including EU, APAC, LATAM, and North America. |
| 2. Are spare parts for the iTero scanner readily available, and what components are most commonly replaced? | Yes. Align Technology maintains a global spare parts logistics network. Commonly replaced components include scan tips (disposable and reusable), handpiece seals, LED modules, and batteries. Distributors should stock critical spares such as scan tips (Type S and Ultra) and handpiece O-rings. As of 2026, all iTero models support modular handpiece servicing, reducing downtime. Registered service partners receive priority access to the Align Technical Support Portal for part ordering and tracking. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of the iTero scanner includes hardware setup, software integration, and clinic workflow calibration. While basic setup can be performed by clinic staff using guided digital onboarding, on-site installation by a certified Align technician is strongly recommended for optimal integration with practice management software (e.g., ClinCheck Pro, Open Dental, Dentrix). The technician ensures DICOM compliance, network configuration, and scanner calibration. Distributors must coordinate installation scheduling through Align’s Partner Success Team within 72 hours of delivery. |
| 4. What warranty coverage is provided with a new iTero scanner purchase in 2026? | All new iTero Element 5 and iTero 5D Plus systems purchased in 2026 include a standard 2-year comprehensive warranty covering parts, labor, and optical calibration. Optional Extended Service Agreements (ESA) are available for up to 5 years, including preventive maintenance, priority technical support, and loaner unit access during repairs. The warranty is non-transferable and requires registration within 30 days of installation. Distributors must ensure end-user registration to activate full coverage. |
| 5. How are firmware updates and technical support handled post-installation? | iTero scanners receive automatic firmware updates via secure cloud connectivity (Align Connect™). Updates are released quarterly and include clinical enhancements, compatibility patches, and security upgrades. Technical support is available 24/7 through the Align Dental Provider Support Center (phone, chat, and remote diagnostics). Registered clinics gain access to the iTero Success Hub, featuring training modules, troubleshooting guides, and live webinars. Distributors are provided with a dedicated Partner Support ID for escalated case management. |
© 2026 Professional Dental Equipment Guide. For authorized distribution use only. Specifications subject to change. Contact Align Technology or your regional distributor for latest technical documentation.
Need a Quote for Itero Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


