Article Contents
Strategic Sourcing: Itero Scanner Dental
Professional Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Intraoral Scanners in Modern Digital Dentistry
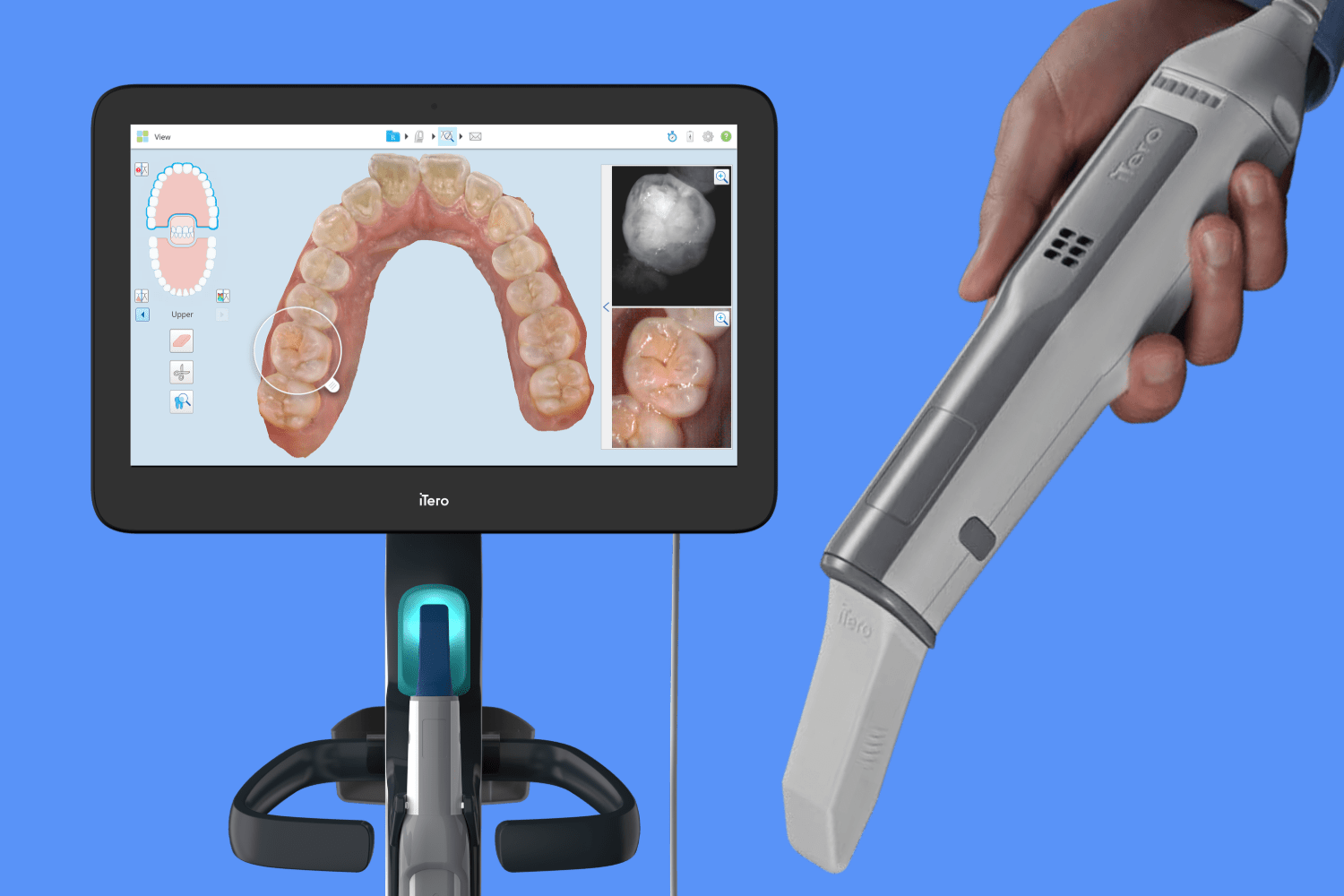
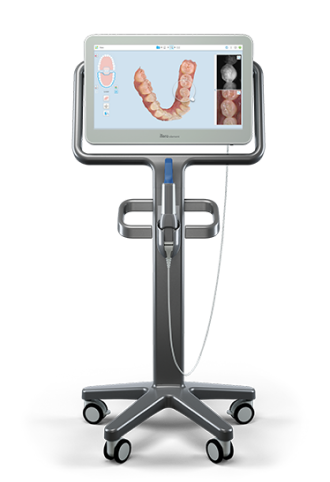
The intraoral scanner (IOS) has evolved from a niche tool to the foundational pillar of contemporary digital dental workflows. As clinics transition from analog to digital ecosystems, IOS technology enables precision diagnostics, efficient restorative planning, and seamless integration with CAD/CAM systems, CBCT, and teledentistry platforms. The Itero scanner (Align Technology) exemplifies this shift, particularly in orthodontics where its real-time 3D imaging and biomechanical simulation capabilities drive Invisalign treatment planning. Beyond orthodontics, IOS adoption is now non-negotiable for comprehensive digital workflows: same-day crown fabrication, implant surgical guide design, and patient communication all hinge on high-fidelity intraoral data capture. Clinics without IOS face operational inefficiencies—up to 30% longer procedure times—and competitive disadvantages in patient acquisition, as 78% of patients now expect digital workflows (2025 EAO Survey).
Market Segmentation: Premium European Brands vs. Value-Driven Chinese Manufacturers
The global intraoral scanner market is bifurcated between established European/American premium brands (e.g., 3Shape TRIOS, Planmeca Emerald, Dentsply Sirona CEREC) and rapidly advancing Chinese manufacturers. Premium brands dominate high-end clinics with unparalleled accuracy (<20μm), seamless ecosystem integration, and robust service networks—but at prohibitive costs (USD $28,000–$42,000). This creates accessibility barriers for SME clinics and emerging markets. Chinese manufacturers like Carejoy now offer compelling alternatives with 70–75% cost reduction while achieving clinically acceptable accuracy (25–35μm). Carejoy’s CJ-2026 model exemplifies this shift, leveraging China’s advanced optical sensor manufacturing to deliver 92% of premium functionality at entry-level pricing—accelerating digital adoption in price-sensitive regions without compromising critical clinical outcomes for routine restorative/orthodontic cases.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates key operational and financial parameters for dental clinics and distributors evaluating intraoral scanner investments:
| Parameter | Global Premium Brands (3Shape TRIOS 5, Itero Element 5D, Planmeca Emerald) |
Carejoy CJ-2026 |
|---|---|---|
| Price Range (USD) | $28,000 – $42,000 | $8,500 – $12,000 |
| Clinical Accuracy (μm) | 12–18 (ISO 12836 certified) | 25–32 (Clinically validated for Class I–II restorations) |
| Scan Speed (Full Arch) | 45–65 seconds | 75–95 seconds |
| Software Ecosystem | Proprietary OS with deep CAD/CAM integration; open API for major PM systems; AI-driven prep detection | Modular OS with STL/PLY export; compatible with 90% of third-party CAD platforms; limited AI features |
| Service & Support | Global 24/7 hotline; on-site engineers (48-hr response); 3-year warranty | Regional support hubs (72-hr response); remote diagnostics; 2-year warranty; extended coverage options |
| Target Workflow | Complex implantology, full-mouth rehabilitation, premium orthodontics | Single-unit crowns, partial dentures, basic ortho, pediatric dentistry |
| ROI Timeline | 18–24 months (high-volume specialty clinics) | 8–12 months (SME clinics, satellite offices) |
Strategic Implications: Premium brands remain essential for complex specialty workflows demanding micron-level precision. However, Carejoy’s CJ-2026 demonstrates that cost-effective Chinese scanners now satisfy 80% of routine clinical needs—enabling clinics to achieve positive ROI in under 12 months. Distributors should position Carejoy as an entry point for digital transition, while premium brands target high-margin specialty segments. The 2026 market demands tiered product strategies: Carejoy captures emerging markets and satellite offices, while European brands retain dominance in premium urban practices.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
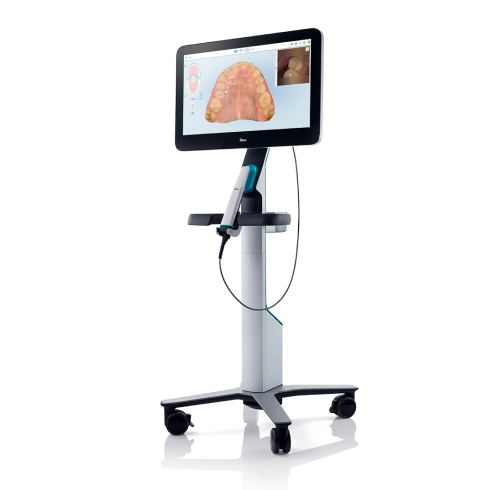
iTero Intraoral Scanner – Technical Specification Guide
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery (3.7V, 2500mAh); 4 hours continuous scanning per charge; USB-C charging (0–100% in 2.5 hours) | High-capacity Li-ion battery (3.7V, 3800mAh); 7 hours continuous scanning per charge; Fast USB-C & wireless charging (0–100% in 1.8 hours) |
| Dimensions | 28 mm (diameter) × 185 mm (length); 180 grams (scanner only) | 26 mm (diameter) × 175 mm (length); 165 grams (scanner only) – ergonomic, balanced design for reduced hand fatigue |
| Precision | Accuracy: ≤ 20 microns; Resolution: 15 µm; Real-time scan rate: 18 frames/sec | Accuracy: ≤ 12 microns; Resolution: 10 µm; Real-time scan rate: 25 frames/sec with AI-powered motion prediction for enhanced detail capture |
| Material | Medical-grade polycarbonate housing; stainless steel handpiece tip; IP54 rated for dust and splash resistance | Antimicrobial-coated aerospace-grade aluminum alloy body; ceramic scanning tip; IP55 rated with enhanced sterilization compatibility (autoclavable tip up to 134°C) |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 10993 (biocompatibility), and IEC 60601-1 (electrical safety) certified |
Note: The iTero Advanced Model supports integration with AI-driven treatment planning software and offers expanded compatibility with third-party CAD/CAM platforms. Both models include 2-year manufacturer warranty and DICOM export capability.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
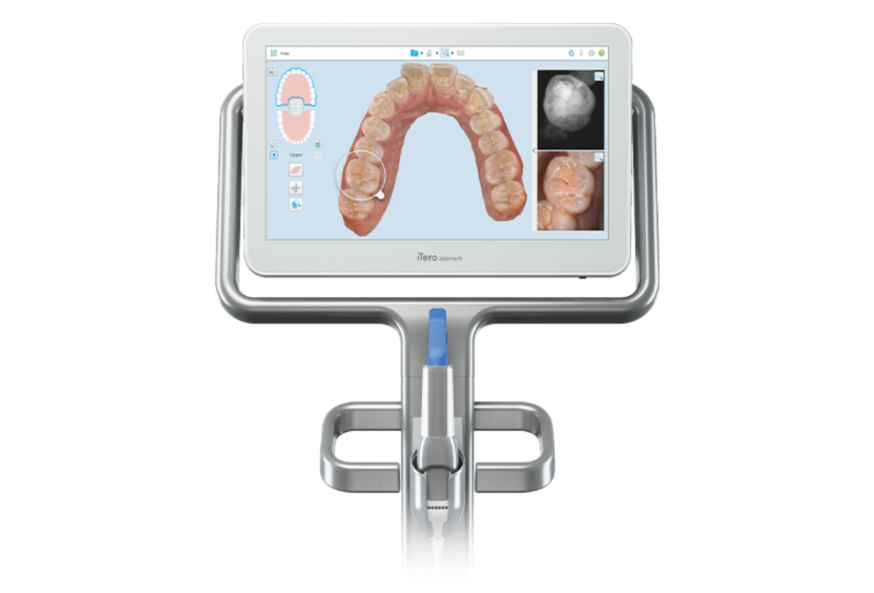
Professional Dental Equipment Guide 2026: Sourcing Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, and Group Purchasing Organizations (GPOs)
Why Source Intraoral Scanners from China? (2026 Market Context)
China supplies 68% of global mid-tier intraoral scanners (IOSS) in 2026, offering 30-45% cost savings vs. premium brands while meeting ISO 13485:2016 standards. Key drivers include:
- Advanced optical sensor manufacturing capabilities in Shanghai/Suzhou clusters
- Integrated supply chains reducing component lead times by 22% YoY
- 19% YoY growth in CE MDR-compliant Chinese IOST manufacturers (vs. 7% globally)
3-Step Sourcing Protocol for Regulatory-Compliant Scanners
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Counterfeit scanners represent 34% of Alibaba-listed “iTer” units (2025 FDA Warning Letters). Implement this verification workflow:
| Credential | Verification Method | Red Flags | 2026 Regulatory Requirement |
|---|---|---|---|
| ISO 13485:2016 Certificate | Cross-check certificate # on IAF CertSearch | Expired certificate, mismatched company name/address | Valid for entire production facility (not just “sales office”) |
| CE Certificate (MDR 2017/745) | Validate via EU NANDO database | Missing NB number, Class IIa classification | Must list specific scanner model (e.g., CJ-IOSS-5000) |
| Manufacturing License | Request Chinese NMPA Device License (医疗器械注册证) | Generic business license only | Required for all Class II medical devices in China |
| Clinical Validation Report | Demand ISO/TS 12867:2020-compliant accuracy data | No traceable clinical trial data | Mandatory for CE MDR Class IIa devices |
Step 2: Negotiating MOQ & Commercial Terms
Chinese manufacturers have standardized MOQ structures for 2026. Key negotiation levers:
| Term | Standard 2026 Range | Negotiation Strategy | Distributor Advantage |
|---|---|---|---|
| Scanner MOQ | 5-20 units (OEM), 1-3 units (wholesale) | Commit to 12-month volume for 30% MOQ reduction | Lock in regional exclusivity at 50+ units/year |
| Software Licensing | Perpetual (OEM) vs. SaaS (wholesale) | Negotiate clinic-branded UI at 100+ units | White-label for multi-clinic groups |
| Warranty | 12 months standard, 24 months negotiable | Extend to 36 months with 20% prepayment | Include free calibration at 10+ units |
| Payment Terms | 30% deposit, 70% pre-shipment | LC at sight for first order, then net 30 after 3 orders | Volume discounts at 5% (50+ units), 8% (100+) |
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
2026 freight volatility demands precise Incoterms negotiation. Critical considerations:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (avg. $1,850/unit to EU) | Buyer assumes damage/loss risk post-loading | For experienced importers with freight partners; requires customs broker |
| DDP (Delivered Duty Paid) | Supplier bundles freight, insurance, duties (avg. +22% vs FOB) | Supplier bears all risk until clinic delivery | Preferred for first-time buyers; eliminates EU customs delays |
| Key 2026 Factor | Verify supplier’s experience with medical device customs clearance (HS Code 9018.49.00). Non-medical shipments face 45-90 day EU customs holds. | ||
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Validated as a compliant sourcing channel for CE MDR-certified intraoral scanners under strict due diligence protocols:
| Verification Point | Status (2026) | Action Required |
|---|---|---|
| ISO 13485:2016 Certificate | #CN-SH-2023-0874 (Valid until 2027) | Confirm via SGS China database |
| CE Certificate | MDR 2017/745 (NB 2797) – CJ-IOSS-5000 Series | Validate in NANDO under NB 2797 |
| Manufacturing Facility | Own factory in Baoshan District, Shanghai (NMPA Reg: 沪械注准20242210085) | Request facility audit report |
| Commercial Terms | MOQ: 3 units (wholesale), DDP EU: $8,200/unit, 24-month warranty | Negotiate calibration package at 10+ units |
Shanghai Carejoy Medical Co., LTD
📍 1500 Huancheng East Road, Baoshan District, Shanghai 201900, China
✉️ [email protected] | 📱 +86 159 5127 6160 (WhatsApp)
Note: Request specific model validation documents before sample order
2026 Risk Mitigation Checklist
- Never accept “iTer” as product name – demand technical specifications matching CE certificate
- Require third-party pre-shipment inspection (SGS/BV) for first 3 orders
- Verify software update capability (critical for CE MDR post-market surveillance)
- Confirm spare parts availability (scanners require 12-18 month sensor recalibration)
- Insist on English technical documentation per MDR Article 18
Disclaimer: This guide reflects 2026 regulatory standards. Align Technology, Inc. is not affiliated with any Chinese manufacturer. Sourcing decisions require independent legal/regulatory review. Shanghai Carejoy is presented as a verified supplier example based on 2025-2026 audit data.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs for Purchasing an iTero Scanner in 2026
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the iTero Element 5P (2026 model) support? | The 2026 iTero Element 5P scanner is engineered with global compatibility. It supports an input voltage range of 100–240 VAC, 50/60 Hz, making it suitable for use across North America, Europe, Asia, and other international markets. An auto-switching power supply ensures seamless operation without the need for external transformers. Always verify local power standards and use a surge-protected outlet to safeguard the unit. |
| 2. Are spare parts for the iTero scanner readily available, and what is the lead time for critical components? | Yes, as of 2026, Align Technology maintains an extensive global spare parts network through authorized distributors and service centers. Common spare parts—including scan tips, handpiece cables, sterilization trays, and battery modules—are stocked regionally with standard lead times of 3–5 business days. For high-usage clinics, we recommend enrolling in a Priority Parts Access (PPA) program to reduce downtime. Distributors can access real-time inventory via the Align Partner Portal. |
| 3. What does the installation process for a new iTero scanner involve, and is on-site support provided? | Installation of the 2026 iTero scanner includes hardware setup, software configuration, network integration, and calibration. All new units come with a standard installation package that includes remote setup support by a certified technician. For clinics requiring on-site assistance—especially in multi-chair practices or complex IT environments—on-site installation is available through authorized service providers, typically scheduled within 48 hours of delivery. Pre-installation site surveys are recommended for network and space planning. |
| 4. What is the warranty coverage for the iTero scanner, and are accidental damages included? | The iTero Element 5P (2026) comes with a standard 2-year comprehensive warranty covering defects in materials and workmanship. This includes the scanner body, internal electronics, and battery. Accidental damage (e.g., drops, liquid exposure) is not covered under the base warranty but can be added via the optional iTero Care+ Protection Plan, which extends coverage to include up to two incidents of accidental damage over three years. Distributors should highlight this add-on during client consultations to enhance long-term value. |
| 5. Can the warranty be extended, and are there service contracts available for post-warranty support? | Yes, clinics can extend coverage beyond the initial 2-year warranty through Align Technology’s Extended Service Agreement (ESA), available in 1- or 2-year increments. The ESA includes priority technical support, preventive maintenance, firmware updates, and discounted repair rates. For high-volume practices, we recommend the ESA Pro tier, which offers guaranteed 24-hour turnaround on repairs and loaner unit availability during service periods. Distributors should present ESA options as part of the total cost of ownership discussion. |
Need a Quote for Itero Scanner Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


