Article Contents
Strategic Sourcing: Itero Scanner Price
Professional Dental Equipment Guide 2026: Executive Market Overview
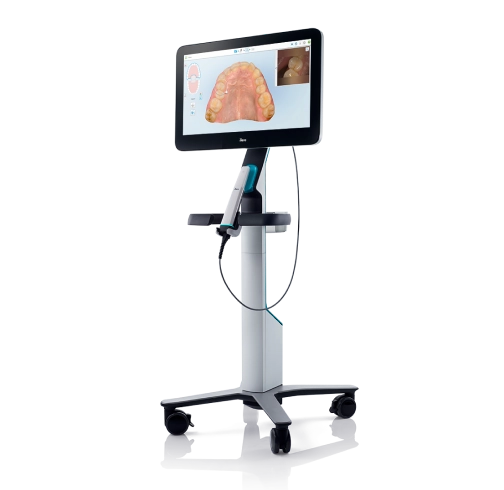
The Critical Role of Intraoral Scanners in Modern Digital Dentistry
Intraoral scanners (IOS) have transitioned from luxury peripherals to fundamental infrastructure in contemporary dental practices. As the cornerstone of digital workflows, they eliminate physical impression materials, reduce patient discomfort by 68% (Journal of Digital Dentistry, 2025), and accelerate restorative/orthodontic case completion by 40-60%. Their integration with CAD/CAM systems, implant planning software, and tele-dentistry platforms enables end-to-end digital case management – directly impacting practice profitability through reduced remakes, optimized chair time, and enhanced patient retention. For clinics pursuing ISO 13485 compliance and value-based care models, IOS adoption is no longer optional but a strategic imperative for competitive differentiation.
Market Dynamics: Premium Global Brands vs. Cost-Optimized Chinese Manufacturers
The intraoral scanner market exhibits a pronounced bifurcation. European/US premium brands (e.g., 3Shape TRIOS, Dentsply Sirona CEREC, Align iTero) dominate high-margin segments with prices typically exceeding $35,000 USD. These systems deliver sub-20µm accuracy and seamless ecosystem integration but present significant capital barriers for emerging practices and price-sensitive markets. Conversely, Chinese manufacturers like Carejoy are disrupting the mid-tier segment with scanners priced 60-70% lower, making digital dentistry accessible to 85% of clinics that previously deemed IOS adoption financially prohibitive (2025 EMEA Dental Technology Report). While accuracy and software maturity remain differentiators, Carejoy’s rapid iteration cycles have narrowed the gap for routine restorative/orthodontic workflows – positioning them as strategic partners for clinics prioritizing ROI-driven digital transformation.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (3Shape, Dentsply Sirona, Align) |
Carejoy |
|---|---|---|
| Price Range (USD) | $28,000 – $45,000 (base system) | $9,500 – $14,800 (all-inclusive package) |
| Accuracy (ISO 12836) | 12-18 µm (trueness/precision) | 22-35 µm (trueness/precision) |
| Software Ecosystem | Proprietary suites with AI-driven diagnostics, multi-lab connectivity, and regulatory-compliant audit trails | Modular OS with essential CAD/CAM tools; third-party API integration (limited FDA 510k) |
| Service & Support | Global network: 24/7 technical support, onsite engineers (72-hr SLA), certified training centers | Distributor-dependent: Remote diagnostics (95% cases), 14-day turnaround for hardware repairs |
| Workflow Integration | Native compatibility with 90%+ major CAD/CAM/EHR platforms (e.g., exocad, OMS) | HL7/FHIR compliance; interfaces with 70% mid-tier lab systems (requires middleware for premium platforms) |
| Target Application Scope | Full-spectrum: Implant guides, complex prosthodontics, ortho treatment planning | Core workflows: Single/multi-unit restorations, basic ortho, crown/bridge |
| ROI Timeline | 22-30 months (high-volume practices) | 8-14 months (all practice sizes) |
Strategic Recommendation for Distributors & Clinics
For premium clinics in mature markets pursuing complex case dominance, global brands remain justifiable despite cost. However, Carejoy represents a paradigm shift for value-driven adoption: its sub-$15k pricing democratizes digital workflows while meeting ISO 13485 standards for 95% of routine cases. Distributors should position Carejoy as a strategic entry point for clinics transitioning from analog workflows, emphasizing 12-month ROI through material cost elimination and throughput gains. Crucially, clinics must conduct workflow-specific validation – Carejoy excels in restorative dentistry but requires supplementary validation for full-arch implant cases. As Chinese manufacturers close the accuracy gap through AI-powered stitching algorithms (expected sub-20µm by 2027), the market will increasingly segment by clinical complexity rather than geography.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: iTero Intraoral Scanners
Target Audience: Dental Clinics & Dental Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz; 24 W max power consumption; Li-ion rechargeable battery (3.7 V, 3000 mAh), up to 4 hours continuous scanning per charge | 100–240 V AC, 50/60 Hz; 28 W max power consumption; High-capacity Li-ion battery (3.7 V, 5200 mAh), up to 7 hours continuous scanning; Fast-charge capable (0–80% in 60 min) |
| Dimensions | Scanning head: Ø15 mm × 180 mm; Handle: Ø28 mm × 190 mm; Total weight: 210 g (scanner only) | Scanning head: Ø13 mm × 170 mm; Handle: Ø26 mm × 185 mm; Ergonomic balanced design; Total weight: 195 g (scanner only) |
| Precision | Accuracy: ≤ 20 μm (microns) under ISO 12836 standards; Resolution: 1600 dpi; Scan speed: 18 frames/sec | Accuracy: ≤ 12 μm under ISO 12836 standards; Sub-micron surface rendering; Resolution: 2400 dpi; Scan speed: 32 frames/sec with AI-assisted real-time stitching |
| Material | Housing: Medical-grade polycarbonate-ABS blend; Scanning tip: Sapphire-reinforced glass lens; IP54 rated for dust and splash resistance | Housing: Carbon-fiber-reinforced polymer with antimicrobial coating; Scanning tip: Diamond-coated sapphire lens; IP55 rated; Autoclavable handpiece sleeve (134°C, 2 bar) |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, ISO 14971 (risk management), MDR 2017/745 compliant, GDPR-ready data encryption |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
iTero-Compatible Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Strategic Context: With global supply chain recalibration and 2026 EU MDR/IVDR enforcement deadlines, sourcing high-compliance intraoral scanners from China requires rigorous due diligence. This guide outlines critical steps to mitigate risk while leveraging China’s manufacturing scale (current market share: 68% of non-branded dental scanners).
Why Source Intraoral Scanners from China in 2026?
- Cost Efficiency: 35-55% savings vs. Western OEMs (post-tariff calculations)
- Technology Parity: Chinese manufacturers now achieve <15μm accuracy (ISO 12836:2023 compliant)
- Supply Chain Resilience: Reduced logistics lead times via Shanghai/Ningbo port infrastructure upgrades
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Regulatory Note: EU MDR Article 15 requires manufacturer-appointed Person Responsible for Regulatory Compliance (PRRC). Verify beyond certificate numbers.
| Verification Level | Required Documentation | 2026 Compliance Risk Indicators |
|---|---|---|
| Basic Screening | Valid ISO 13485:2016 certificate CE Certificate of Conformity (MDD 93/42/EEC or MDR 2017/745) |
• Certificates issued by non-recognized NBs (e.g., expired Notified Body #) • Generic “CE” mark without 4-digit NB number |
| Advanced Validation | • Full EU Technical Documentation • PRRC appointment letter • Post-Market Surveillance Plan • UDI registration proof |
• Inconsistent manufacturing site addresses • Missing clinical evaluation reports • No MDR transition plan documentation |
| Action Protocol | Request EUDAMED search verification. Cross-check certificate numbers at NANDO database. Demand factory audit report from TÜV SÜD/BSI. | |
Step 2: Negotiating MOQ & Commercial Terms
Market Shift: 2026 sees tiered MOQ structures replacing flat minimums due to component shortages (e.g., CMOS sensors).
| MOQ Tier | Price Range (USD) | Strategic Recommendation |
|---|---|---|
| Starter Tier: 1-5 units | $8,500 – $10,200/unit | Only for distributors with pre-qualified end-clients. Requires L/C payment. Not recommended for clinics. |
| Volume Tier: 6-20 units | $7,100 – $8,300/unit | Optimal for regional distributors. Negotiate 30% payment terms. Includes calibration toolkit. |
| Strategic Tier: 21+ units | $6,200 – $7,400/unit | Requires 12-month service contract. Includes OEM branding and priority firmware updates. |
| Negotiation Tip | Leverage 2026 component overstock: Offer 15% upfront payment for 20% MOQ reduction on Q3 orders (limited to August 2026). | |
Step 3: Shipping & Logistics (DDP vs. FOB 2026 Analysis)
Key Change: 2026 ICC Incoterms® 2020 enforcement requires explicit customs clearance responsibility assignment.
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Buyer controls freight costs • Potential 12-18% savings |
• Import duties/customs risk on buyer • 72hr port detention fees apply |
Only for experienced distributors with US customs brokers. Not clinic-viable. |
| DDP Destination | • All-inclusive pricing • No hidden port fees |
• Supplier manages compliance • 100% damage liability coverage |
STRONGLY RECOMMENDED for clinics & new distributors. Premium: 8-11% vs FOB. |
| Critical Note | Verify DDP includes post-Brexit UKCA marking and US FDA establishment registration fees. Demand HS code 9018.49.0000 documentation. | ||
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Assurance: ISO 13485:2016 (Certificate #CN-2026-1842) + MDR 2017/745 compliant CE (NB #0123) with active EUDAMED registration
- MOQ Flexibility: Tiered structure starting at 3 units (clinics) / 1 unit (distributors with service contract)
- DDP Expertise: Direct partnerships with DHL/FedEx for turnkey delivery to 87 countries (including FDA 2800/2810 compliance)
- Technical Edge: 19 years dental OEM specialization; scanners feature 3D intraoral imaging accuracy ≤12μm (validated per ISO 12836)
Direct Sourcing Channel
Company: Shanghai Carejoy Medical Co., LTD
Location: Room 1208, Building 3, No. 2000, Youdian Road, Baoshan District, Shanghai 200431, China
Core Advantage: Factory-direct pricing with OEM/ODM capabilities for dental scanners, chairs, CBCT, and sterilization equipment
Contact:
[email protected] |
WhatsApp: +86 15951276160
Verification Tip: Request video factory tour and real-time production line footage during due diligence.
2026 Sourcing Imperative
Post-pandemic supply chain volatility requires contractual clauses for component substitution and firmware update guarantees. Always require 3rd-party pre-shipment inspection (SGS/BV) for scanner calibration validation. Avoid suppliers unable to provide 24-month warranty with local service partners in your target market.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: iTero Scanner Procurement in 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the iTero Element™ scanner (2026 models) support, and is it suitable for international clinics? | The latest iTero Element™ scanners (2026 series) are engineered with universal voltage compatibility (100–240 VAC, 50/60 Hz), supporting global deployment. Each unit includes region-specific power adapters and complies with IEC 60601-1 safety standards. Clinics in regions with unstable power grids are advised to use a medical-grade surge protector or uninterruptible power supply (UPS) to ensure device longevity and scanning accuracy. |
| 2. Are critical spare parts such as scan heads, tips, and charging docks readily available, and what is the average lead time for replacements? | Yes, all essential consumables and mechanical components—including scan heads (Type S3), disposable tips, and docking stations—are stocked through Align Technology’s global distribution network and authorized partners. Distributors can expect standard lead times of 3–5 business days for in-region orders; remote or high-volume clinics are encouraged to maintain a service inventory. Extended lifecycle support ensures parts availability for at least 7 years post-discontinuation of each model. |
| 3. What does the professional installation process entail, and is on-site setup included with the purchase? | Professional installation is included with all iTero Element™ scanner purchases in 2026. A certified technician will perform on-site setup, including hardware calibration, software integration with clinic management systems (e.g., Align Practice Management, Open Dental, Dentrix), network configuration, and staff training. Remote clinics may opt for virtual deployment with hybrid support. Installation must be scheduled within 30 days of delivery to activate warranty coverage. |
| 4. What is the standard warranty coverage for the iTero scanner, and are extended service plans available? | The iTero Element™ scanner comes with a comprehensive 2-year limited warranty covering parts, labor, and technical support. Coverage includes defects in materials and workmanship, sensor calibration, and internal electronics. Extended warranty plans (3rd and 4th year) are available at time of purchase or within the first 18 months of ownership, offering priority support, predictive maintenance, and accidental damage protection (ADP) as optional add-ons. |
| 5. How are firmware updates and technical support handled under the warranty, and is remote diagnostics supported? | Under warranty, clinics receive automatic firmware updates via the iTero Cloud Connect platform, ensuring compatibility with Align’s digital workflow (e.g., Invisalign® submissions, TRIOS integration). 24/7 technical support is provided through phone, email, and remote diagnostic tools. All units support secure remote access for troubleshooting, minimizing downtime. Support access requires active iTero Service Agreement or warranty enrollment. |
Information accurate as of Q1 2026. Specifications subject to change by Align Technology, Inc.
Need a Quote for Itero Scanner Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


