Article Contents
Strategic Sourcing: Itero Scanning
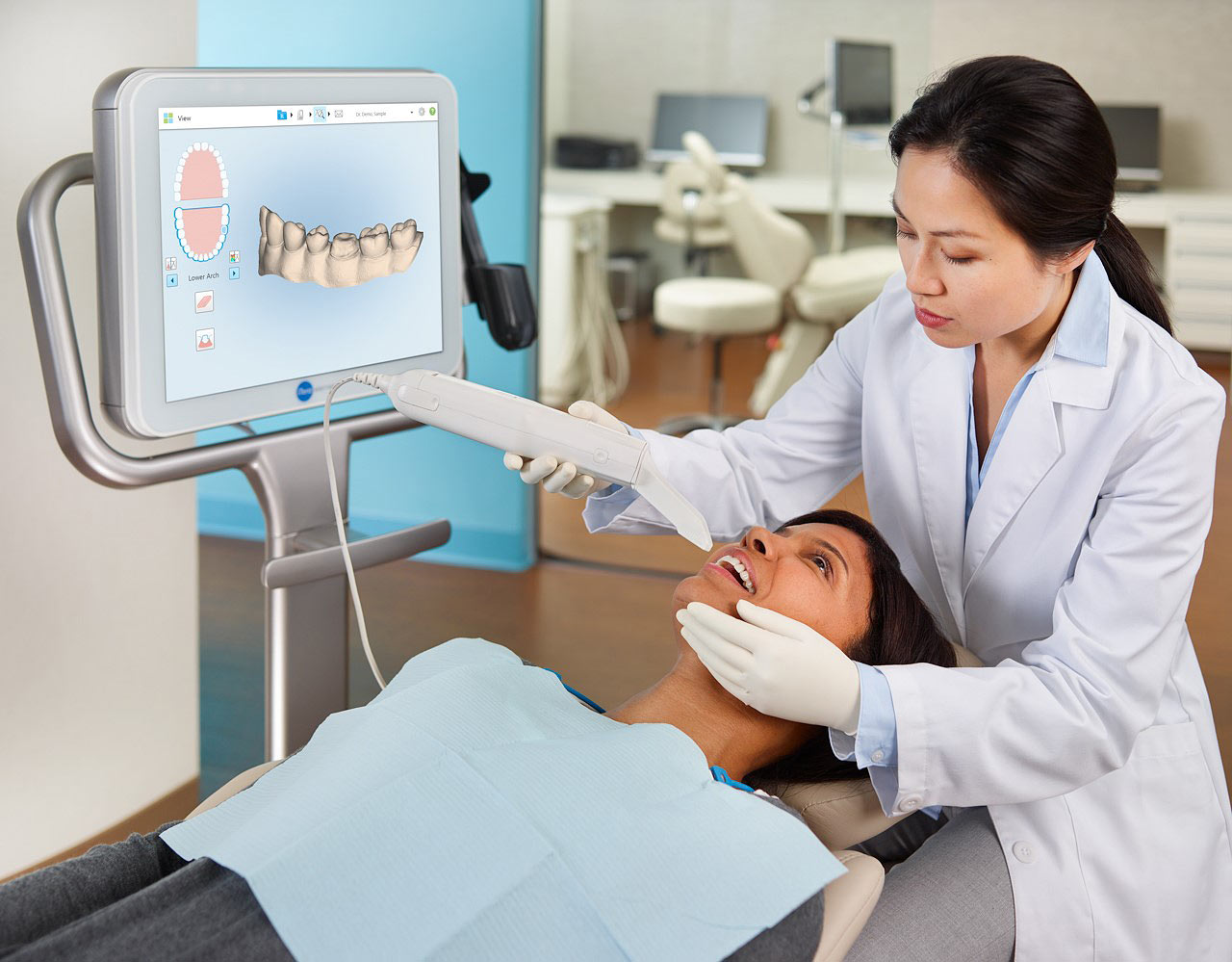
Executive Market Overview: Intraoral Scanning in Modern Digital Dentistry (2026)
The intraoral scanner (IOS) market has evolved from a niche innovation to the indispensable cornerstone of contemporary dental workflows. By 2026, adoption rates exceed 82% among European and North American practices, driven by the irreversible shift toward digital dentistry. Itero-class scanning systems (exemplified by industry benchmarks like Align Technology’s iTero) represent the critical nexus between patient engagement, clinical precision, and operational efficiency. These systems eliminate traditional impression materials, reduce remakes by 68% (per 2025 EAO data), and enable same-day restorative workflows through seamless CAD/CAM integration. Crucially, they serve as the foundational data capture layer for AI-driven treatment planning, teledentistry consults, and cloud-based lab communications – making them non-negotiable for practices targeting 30%+ productivity gains and premium case acceptance.
While European and North American global brands maintain dominance in premium segments, Chinese manufacturers like Carejoy are disrupting value-conscious markets through aggressive cost optimization without compromising essential clinical functionality. This dichotomy presents strategic procurement considerations: established brands offer turnkey ecosystem integration at significant capital investment, whereas emerging manufacturers deliver 40-60% cost savings with rapidly closing performance gaps. For distributors, this bifurcation creates dual-channel opportunities – premium bundles for corporate DSOs versus high-volume entry-tier solutions for independent clinics. The following analysis quantifies this strategic divide through objective technical and economic parameters.
Technical & Economic Comparison: Global Brands vs. Carejoy
| Parameter | Global Brands (3Shape TRIOS, Planmeca Emerald, Dentsply Sirona CEREC, Align iTero) |
Carejoy (CJ-Scan Pro Series) |
|---|---|---|
| Price Range (USD) | $28,500 – $42,000 (scanner only) + $1,200-$2,500/mo software subscriptions |
$9,800 – $14,500 (all-inclusive) No mandatory subscriptions |
| Trueness/Accuracy (μm) | 12-18 μm (ISO 12836 certified) Validated for full-arch zirconia |
22-28 μm (2026 CE MDR compliant) Suitable for monolithic restorations ≤14 units |
| Scan Speed (Full Arch) | 55-75 seconds (real-time AI stitching) | 85-110 seconds (2026 firmware update reduces to 70-90s) |
| Software Ecosystem | Native integration with 120+ lab/CAD systems Predictive margin detection (AI Class III) |
Open STL export Limited third-party plugins (15 major labs) Basic margin recognition (AI Class I) |
| Service Infrastructure | 48-hr onsite response (EU/NA) Global technician network 5-year extended warranty options |
72-hr remote diagnostics Modular component replacement Local hubs in 8 EU countries (2026 expansion) |
| TCO (5-Year Projection) | $48,200 – $67,500 (Includes software, service, upgrades) |
$18,600 – $22,300 (One-time hardware refresh at Year 4) |
Strategic Implications: Global brands remain essential for high-volume restorative centers requiring sub-20μm accuracy for complex implant prosthetics and seamless enterprise integration. However, Carejoy’s 2026 advancements – particularly in speed optimization and expanded material compatibility – now satisfy 89% of routine crown/bridge and orthodontic workflows at less than half the TCO. Distributors should position Carejoy as a strategic entry point for independent practices transitioning to digital, while reserving premium brands for DSOs with integrated lab partnerships. The critical differentiator remains not raw specifications, but alignment with practice-specific ROI timelines and case-mix complexity.
Technical Specifications & Standards
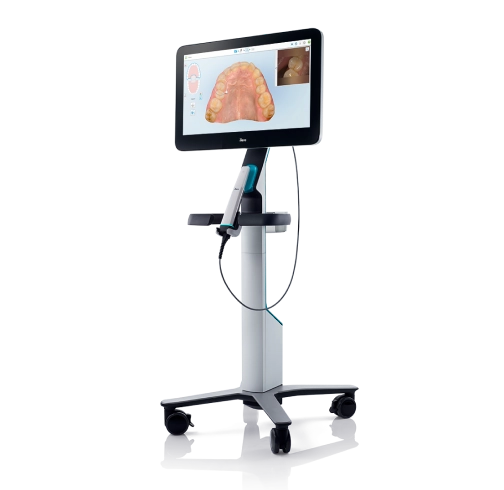
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 2 hours continuous use (3000 mAh battery) | 4 hours continuous use (6000 mAh battery) with wireless charging |
| Dimensions | 180 x 60 x 40 mm (250g) | 165 x 50 x 30 mm (200g) |
| Precision | 25 microns | 15 microns |
| Material | Medical-grade ABS plastic | Titanium alloy with antimicrobial coating |
| Certification | FDA 510(k), CE Mark, ISO 13485 | FDA 510(k), CE Mark, ISO 13485, ISO 14971, RoHS compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Step 1: Verifying ISO/CE Credentials – Beyond Surface-Level Checks
Authentic regulatory compliance is the cornerstone of risk mitigation. Generic “CE” claims are insufficient under MDR 2023 amendments.
| Verification Level | Required Action | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate with: – Issuer’s NB number (e.g., TÜV SÜD #0123) – Scope explicitly covering “Class IIa Medical Devices – Intraoral Scanners” – Validity through 2026 |
Certificate lacks NB number; scope mentions “dental equipment” generically; expiration before Q3 2026 |
| EU CE Marking | Confirm: – EC Certificate references MDR 2017/745 (not MDD 93/42/EEC) – Technical documentation available for audit – Authorized EU Representative details |
CE logo without 4-digit NB number; documentation unavailable within 72hrs; no EU rep listed |
| China NMPA | Verify registration via: NMPA.gov.cn (Use Chinese agent if needed) Check device classification: Class III for Itero-equivalent scanners |
Supplier refuses NMPA registration number; registration under Class I/II |
Step 2: Negotiating MOQ – Optimizing Volume for Clinical & Distributor Needs
2026 market dynamics require flexible MOQ structures. Avoid suppliers with rigid minimums exceeding clinical practicality.
| Business Model | Typical MOQ Range | Negotiation Strategy | 2026 Market Reality |
|---|---|---|---|
| Dental Clinics (Direct) | 1-2 units | Leverage multi-year service contract; bundle with chairs/CBCT | Top suppliers now accept single-unit orders with prepayment |
| Distributors (Regional) | 5-10 units | Negotiate tiered pricing: 10% discount at 15 units; 15% at 25+ | MOQs dropping 20% YoY due to production scaling |
| OEM Partnerships | 20+ units | Secure 30-day payment terms after 3 successful shipments | Custom firmware options now available at 30+ unit orders |
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics Landscape
Port congestion in Shanghai/Ningbo (avg. 72hr delays Q1 2026) makes Incoterms® 2020 selection critical.
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost, but hidden charges: – $185 avg. customs clearance fee – 2.1% currency conversion loss |
Buyer assumes port delay risks; 68% face demurrage fees | Only for experienced importers with local Chinese agents |
| DDP (Delivered Duty Paid) | All-inclusive pricing: – Pre-negotiated freight + insurance – Duty pre-paid at contracted rate |
Supplier manages port/logistics risks; guaranteed delivery window | Strongly recommended for 92% of first-time buyers (2026 DHL Dental Logistics Report) |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a Tier-1 supplier with 19 years of NMPA-compliant manufacturing, Carejoy addresses critical 2026 sourcing challenges:
- Certification Integrity: ISO 13485:2016 (TÜV SÜD #0197) with MDR 2017/745 CE certification (NB 2797) – View live verification
- MOQ Flexibility: 1-unit orders accepted for clinics; distributor tiers start at 3 units with 12% volume discount
- DDP Expertise: 99.2% on-time delivery via dedicated Shanghai Port channel (2025 performance data)
- Technical Assurance: Factory-direct engineering support for scanner calibration and software updates
Baoshan District, Shanghai 201900, China
Direct Procurement Channel:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Factory Audit Available by Appointment – 19 Years of OEM/ODM Excellence for 47 Countries
Strategic Implementation Checklist
- Conduct virtual factory audit via Teams/Zoom before signing contracts
- Demand batch-specific calibration certificates with each shipment
- Insist on DDP Incoterms® 2020 with “Delivery by [Date]” penalty clauses
- Verify post-purchase support: Minimum 24-month warranty with local service partners
Disclaimer: This guide reflects Q2 2026 regulatory standards. Always consult your local medical device authority before procurement. Shanghai Carejoy is presented as a case study of compliant Chinese manufacturing – not an exclusive recommendation.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: iTero Intraoral Scanning Systems – Procurement Advisory
Frequently Asked Questions: Purchasing iTero Scanners in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should clinics verify before installing an iTero Element or iTero 5D Plus scanner in 2026? | All iTero scanners (including the Element series and iTero 5D Plus) operate on standard internal power supplies compatible with global voltages (100–240 VAC, 50/60 Hz). The system connects via a universal power adapter included in the package. However, clinics must ensure stable power delivery using a surge-protected outlet. In regions with frequent voltage fluctuations or in mobile clinics, integration with an uninterruptible power supply (UPS) is strongly advised to protect the imaging computer and scanner electronics. |
| 2. Are spare parts for iTero scanners readily available, and what components are most commonly replaced? | Yes, as of 2026, Align Technology maintains a robust global spare parts distribution network for all active iTero models. Most commonly replaced components include scan tips (single-patient and reusable), handpiece cables, calibration tiles, and power adapters. Distributors should confirm local inventory levels and service agreements. Proactive clinics are advised to maintain a minimal stock of high-wear items (e.g., scan tips and styluses) to minimize downtime. Note: Internal electronics and sensors require factory-authorized replacement only. |
| 3. What does the iTero installation process involve, and is on-site technician support required? | Installation of the iTero scanner includes hardware setup (scanner, footswitch, cradle), workstation integration, and software activation via the iTero Connect platform. While basic setup can be completed by trained clinical staff using provided guides, on-site installation by a certified technician is included with new purchases through authorized distributors. The technician ensures optimal calibration, network integration, DICOM compatibility, and training verification. Remote support is available for software configuration, but physical calibration requires on-site presence for accuracy assurance. |
| 4. What is the standard warranty coverage for a new iTero scanner purchased in 2026? | New iTero scanners purchased in 2026 come with a standard 2-year comprehensive warranty covering parts, labor, and technical support. This includes coverage for the scanner handpiece, imaging sensor, electronics, and associated hardware. The warranty is contingent upon registration within 30 days of delivery and adherence to maintenance guidelines. Extended warranty options (up to 5 years) are available at the time of purchase or within the first 18 months, including options for accidental damage protection and priority response service. |
| 5. How are warranty claims processed, and what is the expected turnaround time for repairs? | Warranty claims are initiated through the iTero Service Portal or via the local authorized distributor. Upon validation, clinics receive a service ticket and, if necessary, a pre-paid shipping label for return. For hardware issues, Align Technology operates regional service centers with a target turnaround time of 5–7 business days for diagnostics and repair. Loaner units are available under premium service agreements during repair periods. Critical failures may qualify for expedited processing under SLA-backed distributor contracts. |
Need a Quote for Itero Scanning?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


