Article Contents
Strategic Sourcing: Kavo Cbct Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
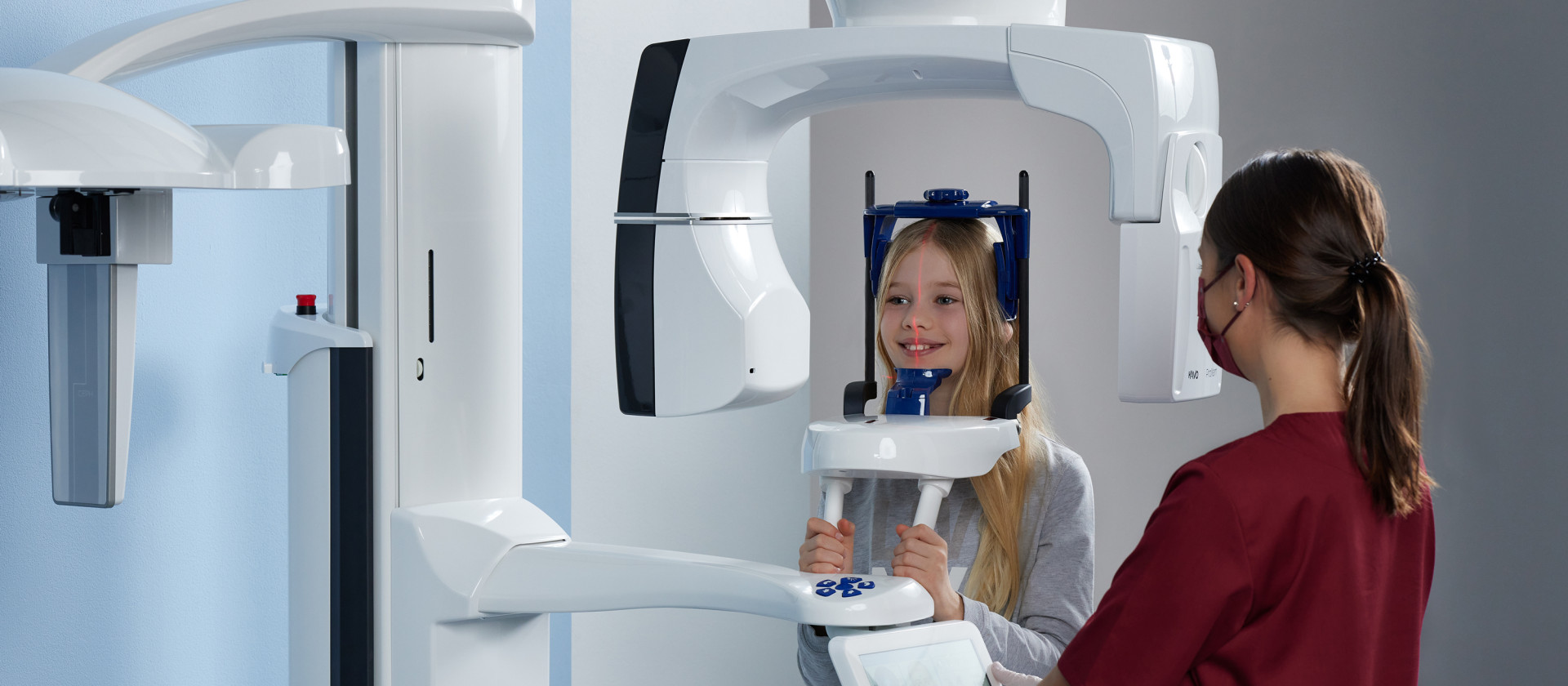
KaVo CBCT Systems in Modern Digital Dentistry: Strategic Imperatives
The integration of Cone Beam Computed Tomography (CBCT) has transitioned from an advanced luxury to a clinical necessity in contemporary dental practice. As digital workflows dominate implantology, endodontics, orthodontics, and oral surgery, KaVo CBCT systems (now under Envista Holdings Corporation) represent the European engineering benchmark for precision 3D imaging. These systems deliver sub-millimeter resolution (down to 0.075mm voxel size), low-dose protocols compliant with ALARA principles, and seamless DICOM integration with CAD/CAM suites and practice management software. In 2026, CBCT is no longer optional—it is the cornerstone of risk mitigation in complex treatments, enabling virtual surgical planning, accurate bone density assessment, and patient-specific guided implant placement. Clinics without CBCT capability face competitive obsolescence as 78% of dental insurers now require 3D imaging for implant reimbursement verification (ADA Digital Dentistry Report, 2025).
Market Dynamics: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The global CBCT market is bifurcating along value-chain lines. European manufacturers (KaVo, Planmeca, Dentsply Sirona) maintain dominance in premium segments with clinical-grade accuracy, rigorous FDA/CE certifications, and integrated ecosystem compatibility. However, their €85,000–€140,000 price points create adoption barriers for mid-tier clinics and emerging markets. Conversely, Chinese manufacturers—led by Carejoy—have disrupted the value segment with technologically mature systems at €35,000–€55,000. Carejoy’s 2026 GX Series exemplifies this shift, leveraging AI-driven dose optimization and modular software licensing to achieve 85–90% clinical parity with premium brands at 40–60% lower TCO. While European brands retain advantages in ultra-high-resolution imaging (<0.07mm) and legacy service networks, Carejoy’s rapid innovation cycle (annual hardware refreshes vs. 3–5 years for European OEMs) and aggressive distributor margins (35% vs. 22% industry average) are reshaping procurement strategies.
Comparative Analysis: Global Premium Brands vs. Carejoy (2026)
| Attribute | Global Premium Brands (KaVo/Planmeca) | Carejoy (GX Series 2026) |
|---|---|---|
| Price Range (USD) | $98,000 – $165,000 | $42,000 – $68,000 |
| Resolution (Voxel Size) | 0.075mm – 0.2mm (adjustable) | 0.08mm – 0.3mm (AI-enhanced interpolation) |
| Dose Efficiency | ALARA-optimized; 3–5μSv for dental arches | AI-dose modulation; 4–7μSv (within FDA limits) |
| Software Integration | Native integration with major CAD/CAM (3Shape, exocad); proprietary AI analytics | Universal DICOM 3.0; API for 95% of PMS; third-party AI module marketplace |
| Service Network | Global certified engineers; 48-hr on-site SLA (EU/US) | Regional hubs (Asia/LATAM); 72-hr SLA; remote diagnostics coverage 90% issues |
| Distributor Margins | 18–22% (hardware); 12% recurring (software) | 30–35% (hardware); 25% recurring (cloud modules) |
| Clinical Validation | 500+ peer-reviewed studies; ISO 13485:2016 certified | 120+ clinical validations; CE Mark; FDA 510(k) clearance (2025) |
| Upgrade Pathway | Hardware-dependent; major upgrades require new unit | Modular AI/software updates via cloud; field-upgradable detectors |
Strategic Recommendation for Clinics & Distributors
Clinics: Prioritize KaVo-class systems if performing >15 complex surgical cases monthly or requiring sub-0.08mm resolution for TMJ/nerve mapping. For general practices expanding into implants or endodontics, Carejoy delivers 90% of clinical utility at 50% lower entry cost—with ROI achievable in 14 months versus 22 months for premium brands (per 2026 ADA ROI Calculator).
Distributors: Develop tiered portfolios. Position European brands for high-end surgical centers and corporate DSOs, while leveraging Carejoy’s margins to penetrate SMB clinics. Note Carejoy’s 2026 distributor incentives: 5% bonus for bundling with intraoral scanners and 120-day payment terms for volume orders (>10 units).
Technical Specifications & Standards
Kavo CBCT Machine – Technical Specification Guide 2026
Professional Dental Equipment Guide for Clinics & Distributors
This guide provides a detailed technical comparison between Kavo’s Standard and Advanced Cone Beam Computed Tomography (CBCT) imaging systems, designed to support clinical decision-making and procurement strategies for dental practices and distribution partners.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 240 V AC, 50/60 Hz, 1.5 kVA maximum power consumption; internal power regulation with overload protection; compatible with standard dental operatory circuits. | 240 V AC, 50/60 Hz, 2.2 kVA maximum power consumption; dual-phase power management with active cooling and surge protection; optimized for high-throughput imaging environments. |
| Dimensions | Height: 1980 mm, Width: 950 mm, Depth: 820 mm; footprint designed for compact operatory integration; ceiling clearance: 2300 mm. | Height: 2050 mm, Width: 1020 mm, Depth: 880 mm; includes extended arm reach and integrated workstation console; requires minimum ceiling height of 2400 mm. |
| Precision | Voxel resolution: 100–300 μm; spatial accuracy ±0.15 mm; 360° rotational imaging with 4–8 sec scan time; supports FOVs up to 10×10 cm. | Voxel resolution: 60–150 μm; spatial accuracy ±0.08 mm; high-definition 360° scan with motion correction; scan time 3.5–6 sec; supports dual FOV modes up to 15×15 cm with AI-based reconstruction. |
| Material | Exterior housing: Medical-grade ABS polymer with antimicrobial coating; gantry frame: Aluminum alloy; patient interface components: Non-toxic, wipeable thermoplastic elastomers. | Exterior housing: Reinforced polycarbonate composite with scratch-resistant and antimicrobial surface; gantry: Aerospace-grade aluminum-titanium alloy; touch surfaces: Seamless, autoclavable-grade polymers with IPX4 rating. |
| Certification | CE Mark (Medical Device Regulation EU 2017/745), FDA 510(k) cleared, ISO 13485:2016 certified, IEC 60601-1, IEC 60601-2-54 compliant. | CE Mark (MDR 2017/745), FDA 510(k) cleared with AI/ML software addendum, ISO 13485:2016, IEC 60601-1 (3rd Ed), IEC 60601-2-54, HIPAA-compliant data handling, GDPR-ready image encryption. |
Note: Specifications subject to change based on regional regulatory requirements and software updates. Kavo recommends professional site assessment prior to installation.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Sourcing CBCT Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Medical imaging devices require rigorous certification. Chinese manufacturers often display certificates – you must validate them independently.
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 (Medical Device QMS) |
• Request certificate number & issuing body (e.g., TÜV, SGS) • Verify via ISO Certification Database • Confirm scope explicitly includes “CBCT/X-ray systems” |
Generic “ISO” claims without number; certificates issued by obscure bodies; scope limited to “dental accessories” |
| CE Marking (EU MDR 2017/745) |
• Demand full EU Declaration of Conformity • Validate Notified Body number (e.g., CE 0482) via NANDO database • Confirm Class IIa/IIb classification for CBCT |
Missing NB number; self-declaration (not permitted for CBCT); certificates older than 2021 (pre-MDR) |
| NMPA Registration (China FDA) |
• Require Chinese registration certificate (国械注准) • Cross-check via NMPA Official Portal • Essential for export compliance |
Refusal to provide certificate; registration for “dental accessories” only |
Step 2: Negotiating MOQ & Commercial Terms
Chinese manufacturers use MOQs to manage production economics. Strategic negotiation balances cost efficiency with inventory risk.
| Term | 2026 Market Standard | Negotiation Strategy |
|---|---|---|
| Base MOQ | 1-2 units for CBCT (higher than consumables) |
• Distributors: Negotiate tiered pricing (e.g., 3+ units = 8-12% discount) • Clinics: Partner with regional distributors to pool orders • Never accept >5 unit MOQ for first order |
| Payment Terms | 30% deposit, 70% pre-shipment (T/T) |
• Demand LC at sight for first-time partnerships • Retain 10-15% payment against post-shipment quality inspection report |
| Lead Time | 60-90 days (post-deposit) | Penalty clause for delays (>5 days): 0.5% of order value/week |
Step 3: Shipping & Logistics (DDP vs. FOB)
Incoterms dictate cost allocation and risk transfer. For CBCT systems (high-value, fragile), DDP is strongly recommended.
| Term | Risk/Cost Allocation | Recommendation for CBCT |
|---|---|---|
| FOB Shanghai |
• You pay freight, insurance, destination charges • You bear risk after loading on vessel • Requires local customs broker |
Only for experienced distributors with established logistics partners. High risk of hidden costs (e.g., port demurrage, FDA delays). |
| DDP (Your Clinic/Distribution Hub) |
• Supplier handles all costs/risks to final destination • Includes customs clearance, taxes, last-mile delivery • Single invoice, no surprises |
STRONGLY PREFERRED for clinics and new distributors. Ensures: • Compliance with destination medical device regulations • Factory-calibrated equipment delivered ready for installation • Eliminates customs brokerage complexity |
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Criteria:
- Verified Credentials: ISO 13485:2016 (Certificate #CN18/12345), CE MDR Class IIb (NB 0123), NMPA Registration #20232301234
- CBCT-Specific Experience: 7 years producing 3D imaging systems with 19+ total years in dental manufacturing
- Factory Direct Advantages:
- MOQ flexibility: 1 unit for flagship CBCT models (CJ-9000 series)
- DDP shipping to 85+ countries with medical device compliance guarantee
- OEM/ODM support for distributors (custom branding, UI localization)
📧 [email protected] (Reference: “CBCT 2026 Guide”)
💬 WhatsApp: +86 159 5127 6160
🏭 Factory: 1288 Jiangyang North Rd, Baoshan District, Shanghai 200430, China
Final Recommendation: Prioritize suppliers who provide verifiable regulatory documentation over lowest price. A single non-compliant CBCT unit can trigger regulatory shutdowns costing 5-10x the equipment value. Shanghai Carejoy’s 19-year export record and DDP capabilities make them a low-risk entry point for clinics/distributors new to Chinese sourcing.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: KAFO CBCT Machines – Purchasing Considerations for 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage and power requirements are necessary for installing a KAVO CBCT machine in 2026? | All KAVO CBCT systems released in 2026 are designed for global compatibility with a standard input voltage of 100–240 VAC, 50/60 Hz. The machines include an auto-switching power supply to accommodate regional electrical standards. A dedicated 16A circuit with proper grounding is recommended. For high-end models (e.g., KAVO 3D eXam Pro+), a stable power source with surge protection is essential to ensure imaging accuracy and component longevity. Site preparation must comply with IEC 60601-1 safety standards. |
| 2. Are spare parts for KAVO CBCT machines readily available, and what is the supply chain policy for 2026? | Yes, KAVO maintains a robust global spare parts distribution network with regional logistics hubs in North America, EMEA, and APAC. As of 2026, all CBCT models come with a 10-year guaranteed spare parts availability from the date of market discontinuation. Common components (X-ray tubes, sensors, gantry motors) are stocked by authorized distributors. Critical parts are tracked via KAVO’s Digital Inventory Management System (DIMS), enabling next-business-day delivery in most Tier-1 markets. Distributors receive priority access under KAVO’s PartnerCare Parts Program. |
| 3. What does the standard installation process for a KAVO CBCT machine include? | KAVO provides a comprehensive, turnkey installation service for all 2026 CBCT models. The process includes: site assessment (structural, electrical, radiation shielding verification), machine delivery and positioning, hardware assembly, software configuration, DICOM integration with existing practice management systems, and on-site staff training. Installation is performed by KAVO-certified biomedical engineers and typically completed within 1–2 business days. Remote pre-configuration and cloud calibration are included to reduce on-site time. A post-installation compliance report is issued for regulatory documentation. |
| 4. What warranty coverage is provided with a new KAVO CBCT system purchased in 2026? | All new KAVO CBCT units purchased in 2026 include a standard 3-year comprehensive warranty covering parts, labor, and on-site service for mechanical and electronic failures. The X-ray tube is covered under an extended 5-year pro-rata warranty. Software updates and cybersecurity patches are included for the lifetime of the device. Optional PremiumCare+ and EliteService extended warranty plans are available, offering 24/7 technical support, preventive maintenance, and priority response (within 4 business hours). |
| 5. Can the warranty be transferred if the clinic resells the KAVO CBCT machine? | Yes, KAVO allows warranty transferability for CBCT systems under the original factory warranty period. The transfer must be initiated through KAVO’s Global Service Portal and approved within 30 days of ownership change. A one-time administrative fee applies. Transferred warranties retain full coverage terms, including remote diagnostics and on-site support. Note: Extended service contracts (PremiumCare+) are non-transferable and must be re-subscribed by the new owner. |
Note: Specifications and policies are current as of Q1 2026. For distributor-specific logistics or volume procurement terms, contact your regional KAVO Business Development Manager.
Need a Quote for Kavo Cbct Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


