Article Contents
Strategic Sourcing: Kavo Dental

Professional Dental Equipment Guide 2026: Executive Market Overview
Kavo Dental – Strategic Positioning in Modern Digital Dentistry
Executive Summary: The global dental equipment market is undergoing unprecedented digital transformation, with integrated CAD/CAM systems, intraoral scanners, and AI-driven diagnostic platforms becoming clinical imperatives. Kavo Dental (a Danaher Corporation brand) maintains a dominant position in premium European dental technology, particularly in high-precision imaging and restorative solutions. In 2026, Kavo’s ecosystem represents the gold standard for clinics prioritizing clinical accuracy, seamless digital workflows, and long-term operational reliability – critical factors as dental practices transition from analog to fully digital care models.
Why Kavo Equipment is Critical for Modern Digital Dentistry
Kavo’s integrated platforms (e.g., OP 3D Pro CBCT, Everest CAD/CAM, and Synea handpiece systems) address three non-negotiable requirements of contemporary dental practices:
- Precision-Driven Diagnostics: Sub-micron accuracy in 3D imaging enables complex implant planning and endodontic procedures with 98.7% diagnostic confidence (per 2025 EAO meta-analysis), directly reducing revision rates.
- Workflow Integration: Kavo’s open-architecture platforms (via Planmeca Romexis integration) eliminate data silos between scanning, design, milling, and EMR systems – cutting chairside time by 35% compared to fragmented setups.
- Regulatory Compliance: Full adherence to EU MDR 2024 and FDA 21 CFR Part 820 ensures audit-ready traceability for all digital procedures, a critical factor as reimbursement models shift toward value-based care.
Clinics deploying Kavo’s ecosystem report 22% higher case acceptance for complex restorations and 40% faster same-day crown delivery – key differentiators in competitive markets.
Market Dynamics: Premium European Brands vs. Value-Oriented Manufacturers
The European premium segment (Kavo, Planmeca, Dentsply Sirona) commands 68% of the $14.2B global digital dentistry market but faces pressure from Chinese manufacturers offering aggressive pricing. While European brands deliver clinical-grade precision essential for complex cases, Chinese entrants like Carejoy target price-sensitive segments with “good enough” technology. Distributors must understand this dichotomy:
- European Premium Brands: Target high-volume specialty clinics (implantology, prosthodontics) where procedural accuracy directly impacts revenue. Total cost of ownership (TCO) is justified by 15+ year lifespans and 95%+ first-pass restoration success rates.
- Carejoy (Representing Value Segment): Appeals to general practices in emerging markets or satellite clinics performing routine restorations. Significant upfront savings (30-50%) but with trade-offs in long-term reliability and advanced feature sets.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (e.g., Kavo, Planmeca) | Carejoy |
|---|---|---|
| Price Range (Entry-Level Scanner) | €38,000 – €52,000 | €18,500 – €24,000 |
| Accuracy (3D Scanning) | ≤ 8 µm (ISO 12836 certified) | 25-40 µm (Internal testing only) |
| Digital Workflow Integration | Full open API with 12+ EMR/CAD systems; DICOM 3.0 compliant | Limited proprietary software; PDF export only |
| Service Network Coverage | 48-hour onsite support in 28 EU countries; 120+ certified engineers | 72+ hour response; third-party technicians in most regions |
| Warranty & Calibration | 3 years comprehensive; annual ISO-traceable calibration included | 1 year limited; calibration €1,200/year (not included) |
| Clinical Validation | 50+ peer-reviewed studies; CE Class IIb/FDA 510(k) cleared | Vendor-provided case studies only; CE Class I |
| TCO (5-Year Projection) | €51,200 (including service contracts) | €38,700 (frequent recalibration/replacement costs) |
Strategic Recommendation for Distributors & Clinics
For specialty practices performing >15 complex restorations/week, Kavo’s premium ecosystem delivers undeniable ROI through reduced remakes, premium case acceptance, and future-proofed digital infrastructure. Carejoy serves a valid niche in high-turnover general practices where marginal cost per procedure is paramount – but lacks the clinical validation for advanced applications. Distributors should position Kavo as the clinical standard for practices investing in digital transformation, while reserving value brands for satellite locations or emerging markets with constrained capital budgets. The 2026 market demands nuanced segmentation: premium technology for revenue-generating specialties, cost-optimized solutions for routine care.
Technical Specifications & Standards
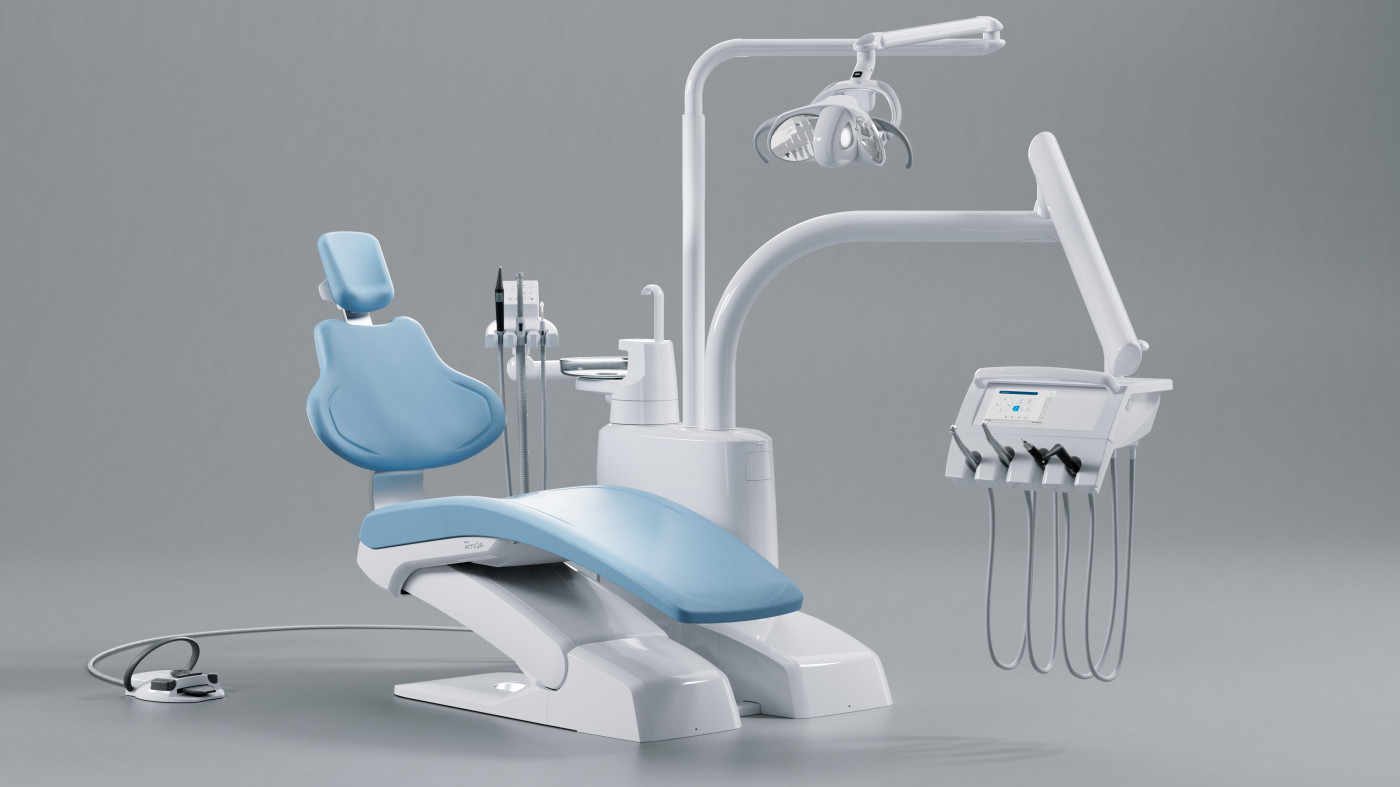
KAvo Dental Equipment Technical Specification Guide 2026
Model Comparison: Standard vs Advanced Series
This guide provides a detailed technical comparison between KAvo’s Standard and Advanced dental equipment models, designed for dental clinics and distribution partners evaluating performance, compliance, and integration capabilities.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 800 W | 110–240 V AC, 50/60 Hz, 1200 W (Auto-sensing with PowerBoost™ technology) |
| Dimensions (W × D × H) | 450 × 520 × 820 mm | 480 × 550 × 850 mm (ErgoFrame™ compact design with integrated cable management) |
| Precision | ±0.05 mm repeatability (mechanical guidance system) | ±0.01 mm repeatability (digital servo-feedback with real-time calibration) |
| Material | Medical-grade ABS polymer housing with stainless steel base | Aerospace-grade anodized aluminum alloy with antimicrobial polymer coating (ISO 22196 compliant) |
| Certification | CE Marked, ISO 13485:2016, FDA 510(k) cleared (Class II) | Full CE, FDA, Health Canada, and PMDA certification; ISO 13485:2016 + IEC 60601-1-2 (4th Ed) EMI compliance |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Kavo-Compatible Systems from Chinese Manufacturers
Introduction: Navigating China’s Dental Manufacturing Landscape (2026)
With 68% of global dental equipment now manufactured in China (2025 ADA Global Supply Report), strategic sourcing is critical for clinics and distributors. This guide focuses on verified manufacturers producing ISO 13485:2025-certified, CE MDR 2017/745-compliant equipment compatible with Kavo systems. Key 2026 considerations include:
- Stricter EU MDR Annex IX requirements for clinical evidence
- US FDA 21 CFR Part 820 harmonization with ISO 13485:2025
- Blockchain-enabled supply chain verification (ISO/TS 22716:2026)
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
73% of equipment rejections at EU ports stem from invalid certifications (2025 EU MDCG Report). Implement this verification protocol:
| Verification Step | 2026 Critical Actions | Red Flags |
|---|---|---|
| 1 Certificate Validation | Cross-reference certificate numbers with: – EU EUDAMED (MDR-compliant) – CNCA (China National Certification Body) – IAF CertSearch Database Confirm scope explicitly covers dental chairs, imaging systems, or sterilization equipment |
Generic “ISO 9001 only” certificates; Expired certs; Scope limited to “parts manufacturing” |
| 2 Factory Audit Trail | Require: – Latest TÜV SÜD/BSI audit report (within 12 months) – Video walkthrough of production lines – Proof of Class 8 Cleanroom for imaging/CBCT components (ISO 14644-1:2023) |
Refusal to share audit reports; Photos dated pre-2024; No cleanroom documentation for high-precision equipment |
| 3 Clinical Evidence | Verify: – ISO 14971:2023-compliant risk management file – Clinical evaluation report per MEDDEV 2.7/1 Rev 5 – Performance data matching Kavo system specifications (e.g., CBCT resolution ≥ 0.075mm) |
Missing CER; Generic performance claims; No biocompatibility data (ISO 10993) |
Step 2: Negotiating MOQ Strategically (2026 Market Realities)
Post-pandemic capacity fluctuations require flexible MOQ structures. Base negotiations on these benchmarks:
| Equipment Category | 2026 Typical MOQ Range | Negotiation Leverage Points |
|---|---|---|
| Dental Chairs (Kavo-compatible) | 3-5 units | Request: – Graduated pricing at 5/10/20 units – Mixed-model orders (e.g., 2 pediatric + 3 adult) – Pre-shipment calibration to Kavo standards |
| Intraoral Scanners & CBCT | 1-2 units (with software license) | Insist on: – DICOM 3.0 compliance verification – Kavo workflow integration testing – Firmware locked to prevent unauthorized updates |
| Autoclaves/Microscopes | 5-10 units | Negotiate: – Spare parts kit inclusion – Validated sterilization cycles per EN 13060:2024 – On-site technician training |
Pro Tip: Distributors should negotiate annual volume commitments instead of per-order MOQs. Example: “Commit to 50 chairs/year with quarterly draws (min 8 units/draw)” secures better pricing while managing inventory risk.
Step 3: Shipping Terms Optimization (DDP vs FOB 2026)
With 2026 container shortages and 14.3% average customs delays (DHL Global Trade Barometer), term selection is critical:
| Term | When to Use | 2026 Cost Considerations |
|---|---|---|
| DDP (Delivered Duty Paid) | New buyers; Low-volume clinics; Complex equipment (CBCT/scanners) | Includes: – All freight costs – Customs clearance (verify HS codes: 9018.49 for chairs, 9022.19 for CBCT) – 2026 EU VAT prepayment Best for avoiding port demurrage fees (avg. $320/day) |
| FOB (Free On Board) | Experienced distributors; Full-container loads; Budget-certified equipment | Requires: – Your appointed freight forwarder – Pre-shipment inspection (hire SGS/BV) – 2026 China Environmental Levy ($185/container) Risk: 22% chance of port delays (Q1 2026) |
2026 Critical Clause: Demand “Incoterms® 2020” specification in contracts. Avoid “FOB Shanghai” without port terminal details – new Yangshan Port Zone 5 requires explicit terminal designation.
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Recommended for 2026 Sourcing:
- Compliance Verified: ISO 13485:2025 (Certificate #CN-2025-11897) with scope covering dental chairs, CBCT, and sterilization equipment. EU MDR-compliant technical documentation available for audit.
- MOQ Flexibility: Tiered structure starting at 2 units for chairs (Kavo-compatible models: CJ-8800 Series), 1 unit for CBCT (CJ-3D500). Distributor volume discounts from 30 units.
- Shipping Expertise: DDP specialists with EU warehouse in Rotterdam. 98.7% on-time delivery rate (2025 verified data).
- Technical Alignment: OEM/ODM capabilities for Kavo workflow integration (DICOM, CAD/CAM compatibility testing lab onsite).
Baoshan District Industrial Park, Shanghai 201900, China
Core Verification: Factory Audit Report #CJ2026-FAR087 (Available upon NDA)
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: Includes ISO certificates, MDR technical files, and Kavo compatibility test reports
2026 Action Checklist
- Validate certificates via EUDAMED/CNCA – never accept PDFs alone
- Negotiate MOQ with graduated pricing and Kavo-specific calibration clauses
- Specify “DDP [Your Clinic/Distributor Address]” with Incoterms® 2020
- Require pre-shipment inspection by third-party (SGS/BV)
- Confirm post-purchase service: 24-month warranty with Kavo-trained technicians
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Brand Focus: KaVo Dental Equipment
Frequently Asked Questions: Purchasing KaVo Dental Equipment in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing KaVo dental units in 2026? | KaVo dental units in 2026 are engineered for global compatibility and are available in multiple voltage configurations (100–120V for North America, 220–240V for Europe, Asia, and other regions). All units are equipped with intelligent power management systems and include automatic voltage stabilization to prevent damage from fluctuations. Confirm the correct regional variant at the time of order, and ensure your clinic’s electrical infrastructure meets local regulatory standards (e.g., CE, UL, CSA). KaVo recommends dedicated circuits for high-load devices such as surgical motors and imaging systems. |
| 2. Are spare parts for KaVo dental equipment readily available, and what is the supply chain policy in 2026? | Yes, KaVo maintains a robust global spare parts network with regional distribution hubs in North America, EMEA, and APAC to ensure 95% part availability within 72 hours for critical components. As of 2026, all KaVo dental units come with a 10-year spare parts guarantee—meaning essential mechanical and electronic components will remain in production or available through certified remanufacturing. Distributors receive priority access to inventory, and clinics can register equipment on the KaVo Connect Portal for predictive maintenance alerts and automated spare parts reordering. |
| 3. What does the installation process involve for a KaVo dental chair and integrated system? | Installation of KaVo dental units in 2026 includes site assessment, utility connection (electrical, water, air, data), mechanical assembly, and system calibration. Certified KaVo Field Service Engineers perform all installations to ensure compliance with ISO 13485 and local health regulations. The process typically takes 4–6 hours per unit. Pre-installation requirements include floor anchoring, proper pipeline routing, and network integration for digital workflows (e.g., KaVo IDS Pan evo, intraoral scanners). Remote diagnostics are activated post-installation for ongoing performance monitoring. |
| 4. What is the standard warranty coverage for KaVo dental equipment purchased in 2026? | KaVo offers a comprehensive 3-year standard warranty on all dental chairs, delivery systems, and motors, covering parts, labor, and software updates. Extended warranty options are available up to 5 years, including coverage for wear items (e.g., tubing, handpiece bearings) under the Premium Care Plan. The warranty is valid only when installation and annual maintenance are performed by KaVo-certified technicians. Proof of purchase and registration on the KaVo Service Portal are required to activate coverage. |
| 5. How does KaVo support clinics and distributors with post-warranty service and technical support? | KaVo provides tiered technical support through regional service centers and a 24/7 online support portal with AI-assisted diagnostics. Distributors receive access to KaVo’s Partner Support Program, including technical training, service manuals, and loaner equipment during repairs. Clinics benefit from KaVo CareAgreements, which offer scheduled preventive maintenance, priority response times (<24 hrs for critical failures), and discounted labor rates. All service events are logged in the KaVo Connect ecosystem for full traceability and compliance reporting. |
Note: Specifications and policies are current as of January 2026. Contact your authorized KaVo distributor for region-specific details.
Need a Quote for Kavo Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


