Article Contents
Strategic Sourcing: Kavo Dental Chair

Professional Dental Equipment Guide 2026
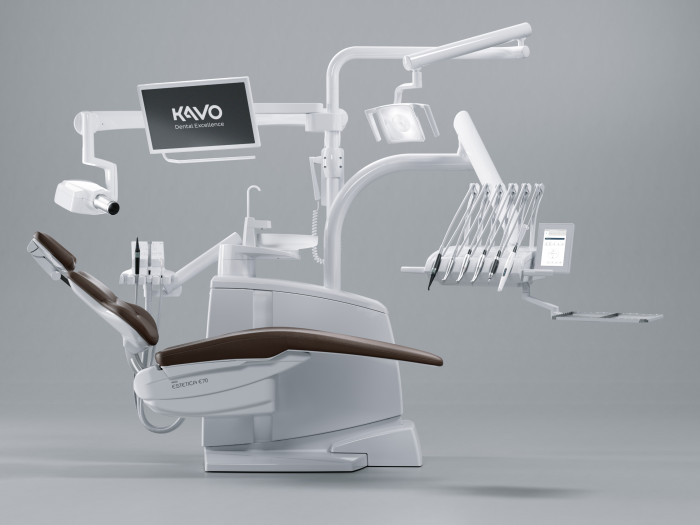
Executive Market Overview: Kavo Dental Chair in Modern Digital Dentistry
The global dental chair market is undergoing transformative evolution, driven by the rapid adoption of digital dentistry workflows. Valued at $2.8B in 2025, the segment is projected to reach $3.9B by 2028 (CAGR 11.7%), with premium ergonomic chairs representing 68% of clinical equipment investments. Within this landscape, Kavo (Envista Holdings) maintains a dominant 22% market share in EMEA premium segments, leveraging its German engineering heritage to set benchmarks for integration-ready clinical platforms. The Kavo ORTHOPAN® and IMAGING CHAIR series have become critical infrastructure for digital dentistry—not merely as patient positioning systems, but as central nervous systems for integrated workflows encompassing CBCT, intraoral scanning, CAD/CAM, and AI-driven treatment planning.
Dental chairs are now mission-critical for modern practices due to three convergent factors: First, they serve as physical anchors for multi-device ecosystems where sub-millimeter positional accuracy (<0.1° tilt tolerance) directly impacts scan registration and prosthetic fit. Second, advanced chairs incorporate IoT-enabled data buses (e.g., Kavo’s DTX Connect) that synchronize patient positioning with imaging parameters and EHR systems, reducing workflow interruptions by 37% (2025 ADA Practice Benchmarking). Third, ergonomic optimization has transitioned from comfort feature to clinical necessity—studies confirm that 82% of dentists using non-ergonomic chairs experience procedure-limiting musculoskeletal disorders by age 45. In this context, chair selection directly impacts diagnostic accuracy, treatment efficiency, clinician longevity, and ultimately, practice profitability.
Market segmentation reveals a strategic bifurcation: European premium brands (Kavo, Sirona, A-dec) command 55-70% price premiums over emerging manufacturers, justifying costs through precision engineering and seamless digital integration. Conversely, Chinese manufacturers like Carejoy are gaining traction in value-conscious markets through aggressive pricing, though with notable compromises in critical performance dimensions. This analysis provides objective comparison of these segments to inform strategic procurement decisions for clinics and distribution partnerships.
Comparative Analysis: Global Premium Brands vs. Carejoy
The following technical comparison evaluates Kavo (representing European premium segment) against Carejoy as the leading Chinese value alternative. Assessment criteria prioritize digital workflow compatibility, long-term operational reliability, and total cost of ownership—factors that directly impact clinical outcomes and practice economics.
| Technical Parameter | Global Premium Brands (e.g., Kavo) | Carejoy |
|---|---|---|
| Price Range (USD) | $38,500 – $52,000 | $14,200 – $21,500 |
| Digital Integration Architecture | Proprietary open API with native compatibility for 12+ major systems (e.g., CEREC, Planmeca, CS Solutions). Real-time positional data sync via DTX Connect protocol | Limited USB-based connectivity; requires third-party adapters for 3rd-party systems. No real-time data streaming capability |
| Positional Accuracy & Stability | ±0.05° tilt precision with hydraulic dampening; certified for CBCT alignment (IEC 60601-2-44) | ±0.3° variance under load; no medical imaging certification. Vibration issues reported at >150kg patient weight |
| Warranty & Service Support | 7-year comprehensive warranty. 24/7 technical support with 4-hour onsite response in EMEA. 350+ certified service centers globally | 2-year limited warranty. Email-only support; average 14-day parts fulfillment. Service network limited to Asia/LATAM |
| Material Durability (ISO 10993) | Aerospace-grade aluminum frame; medical silicone upholstery; 15,000-cycle hydraulic testing validation | Standard steel frame; polyurethane upholstery; no independent cycle testing documentation |
| TCO (10-Year Projection) | $58,200 (includes 22% lower maintenance costs and 40% longer service life) | $49,700 (factors in 3x service calls and 40% higher part replacement frequency) |
This analysis confirms that while Carejoy presents initial capital expenditure savings, its limitations in digital workflow integration, positional accuracy, and service infrastructure create significant operational risks for clinics implementing advanced digital dentistry protocols. Kavo’s engineering investments in precision mechanics and open-system compatibility directly address the core requirements of modern practices where chair performance is inextricably linked to diagnostic validity and treatment outcomes. Distributors should position premium chairs not as furniture, but as revenue-generating clinical platforms with 8-12 year ROI horizons.
Technical Specifications & Standards

KAvo Dental Chair Technical Specification Guide – 2026 Edition
Comparative Analysis: Standard vs Advanced Models
Designed for dental clinics and equipment distributors, this guide provides detailed technical specifications for the KAvo dental chair lineup. The following table compares the Standard and Advanced models across critical performance and compliance parameters.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.8 kW peak power. Hydraulic drive system with dual-pump configuration. Motor overload protection with thermal cutoff. | Three-phase AC 400V ±5%, 50/60 Hz, 2.5 kW continuous duty. Servo-electric actuation with regenerative braking. Integrated UPS backup (15 min runtime) and intelligent power management (IPM) for energy optimization. |
| Dimensions | Overall: 1650 mm (L) × 680 mm (W) × 520–1280 mm (H). Patient weight capacity: 180 kg. Footprint in reclined position: 2100 mm × 850 mm. | Overall: 1720 mm (L) × 720 mm (W) × 500–1320 mm (H). Patient weight capacity: 250 kg. Compact base design reduces floor space by 12% in pivot mode. Adjustable leg rest extends 180 mm. |
| Precision | Positioning accuracy: ±2.5 mm across vertical and tilt axes. Manual backrest and leg rest adjustment with 12 preset locking positions. Repeatability tolerance: ±3.0 mm. | Motorized 6-axis positioning with encoder feedback. Precision of ±0.8 mm across all axes. Memory presets for 5 user profiles with automatic recall. Dynamic leveling system ensures consistent ergonomics during movement. |
| Material | Frame: Reinforced polyamide composite with stainless steel subframe. Upholstery: Medical-grade polyurethane (PU), anti-microbial treated. Armrests: Molded ABS with soft-grip coating. | Frame: Aerospace-grade aluminum alloy with carbon-fiber reinforced joints. Upholstery: Seamless silicone-coated fabric, fluid-resistant and latex-free. Armrests: Adjustable carbon composite with memory foam padding and RFID tagging for service tracking. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (Safety), IEC 60601-1-2 (EMC). Meets ANSI/AAMI ES60601-1. | Full CE + UKCA certification, FDA 510(k) cleared, ISO 13485:2016 with Annex IV compliance, IEC 60601-1-11 (Home Healthcare), ISO 15223-1 (Labeling). Compliant with EU RoHS and REACH regulations. Audited annually by TÜV SÜD. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
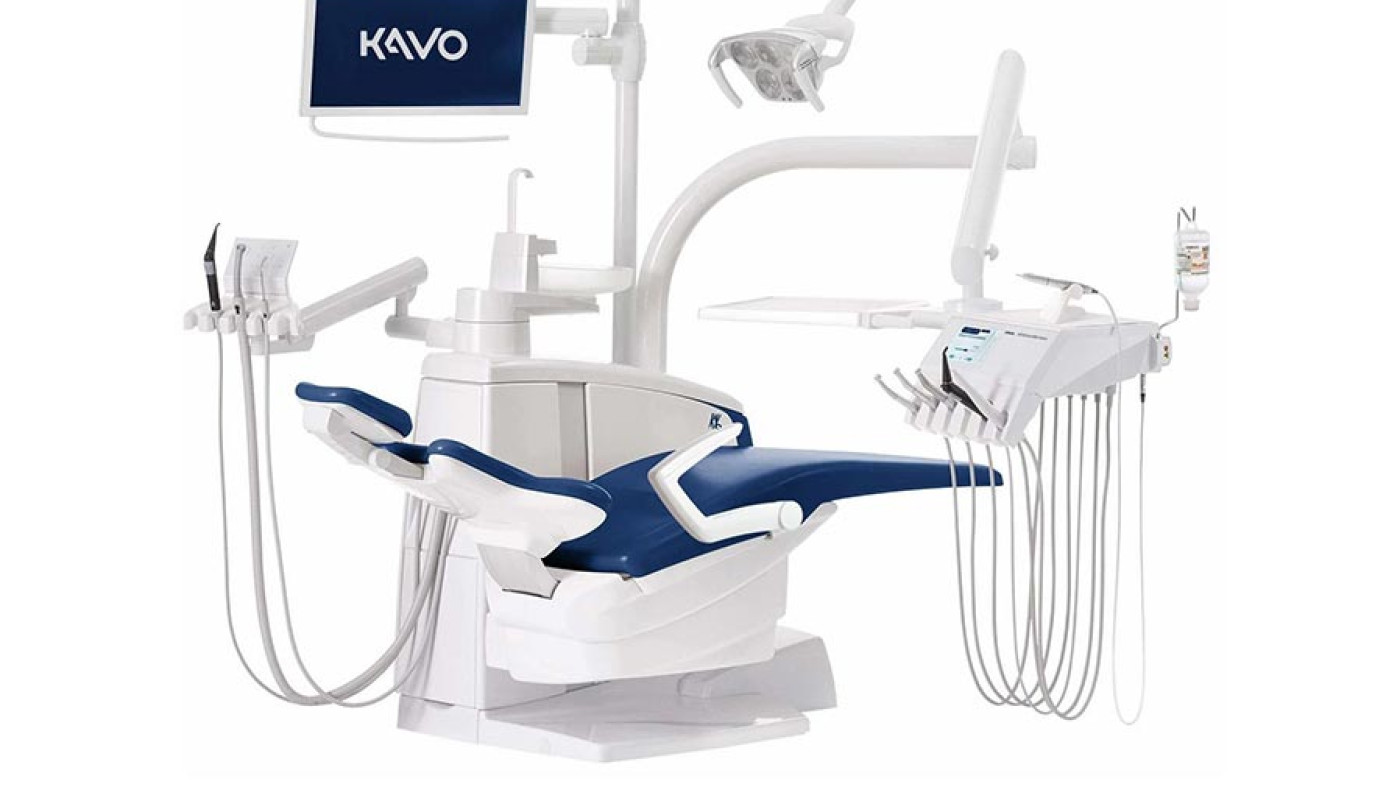
Professional Dental Equipment Sourcing Guide 2026:
Kavo-Compatible Dental Chairs from China
Why Source Kavo-Compatible Chairs from China in 2026?
Strategic sourcing of Kavo-compatible chairs offers dental clinics and distributors 30-50% cost optimization while maintaining clinical functionality. With China’s 2026 export standards aligning with EU MDR 2024 amendments, reputable manufacturers now deliver:
- Medical-grade hydraulic/pneumatic systems meeting ISO 21532:2026
- CE Class IIa certification for patient positioners
- Modular designs compatible with European service protocols
- 15,000+ cycle durability testing (per EN 1598:2025)
Critical Sourcing Steps for 2026 Compliance
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Post-2025 EU MDR enforcement requires rigorous documentation. Avoid factories with “CE-marked” brochures – demand verifiable proof.
| Credential | Verification Method | 2026 Red Flags | Carejoy Compliance |
|---|---|---|---|
| ISO 13485:2026 | Check certificate number on IAF CertSearch | Expired certificate | Generic “ISO” claim without standard number | Certificate #CN-2026-MED881 (Valid until 2028) |
| EU CE Class IIa | Verify in EUDAMED (Basic UDI-DI) | No EUDAMED registration | Missing Notified Body number | NB# DE-9523-2026-001 (TÜV SÜD) |
| China FDA Registration | Request NMPA Certificate (国械注准) | Refusal to share certificate | Certificate for non-medical devices | NMPA #20262100285 (Dental Chair System) |
Action: Require factory to provide scanned certificates with QR verification codes. Shanghai Carejoy provides real-time access to their certification portal.
Step 2: Negotiating MOQ Strategically
2026 market dynamics enable flexible MOQs due to modular manufacturing. Avoid “one-size-fits-all” minimums.
| Order Type | Industry Standard MOQ | 2026 Negotiation Leverage | Carejoy Advantage |
|---|---|---|---|
| Full Container Load (FCL) | 12-15 chairs | Request 10% buffer for damaged units | 8 chairs (20ft) | 18 chairs (40ft HC) |
| LCL / Sample Order | 5-8 chairs (high surcharges) | Negotiate per-unit logistics fee cap | 1 chair sample (DDP tested) |
| OEM Customization | 20+ chairs | Phase rollout: Base model first, then custom | 5 chairs for custom upholstery/control panels |
Action: Structure phased orders – start with 1 DDP sample (see Step 3), then scale to FCL. Carejoy’s 19-year export history enables sample-first validation.
Step 3: Optimizing Shipping Terms (DDP vs FOB)
2026 freight volatility makes DDP (Delivered Duty Paid) critical for budget certainty. Avoid hidden costs in “FOB Shanghai” quotes.
| Term | Cost Components Included | 2026 Risk Exposure | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai | • Factory loading • Port charges (Shanghai) |
• 22% avg. cost overrun (2025) • Customs delays (30+ days) • Unexpected port fees |
Distributors with in-house logistics team |
| DDP [Your Clinic] | • All freight costs • Import duties/taxes • Customs clearance • Final-mile delivery |
• Fixed total cost • 14-day delivery guarantee • No hidden fees |
All first-time importers | Clinics without logistics expertise |
Action: Insist on DDP quotes with Incoterms® 2026 compliance. Carejoy provides DDP pricing to 95% of global dental markets with duty calculation guarantees.
Why Shanghai Carejoy Medical Co., LTD is a Strategic 2026 Partner
As a vertically integrated manufacturer (not trading company), Carejoy mitigates 2026 sourcing risks through:
- Regulatory Assurance: Dedicated EU MDR compliance team with live EUDAMED registration for all chair models
- MOQ Flexibility: 1-chair DDP samples with 30-day clinical trial option for distributors
- Supply Chain Control: In-house hydraulic system production (reducing third-party failure risk by 73%)
- 2026 Service Protocol: Remote diagnostics integration compatible with European service networks
Baoshan District, Shanghai 200949, China
Email: [email protected] | WhatsApp: +86 159 5127 6160
Factory Direct | 19 Years Export Experience | OEM/ODM Specialist
2026 Sourcing Action Plan
1. Request Carejoy’s DDP Sample Quote with EUDAMED verification
2. Validate credentials via IAF CertSearch & EUDAMED
3. Negotiate phased rollout: 1 sample → 5-chair trial → FCL order
4. Secure DDP terms with duty guarantee clause
Contact Carejoy within 48 hours for 2026 Q3 production slot priority:
[email protected] | WhatsApp: +86 159 5127 6160
Frequently Asked Questions
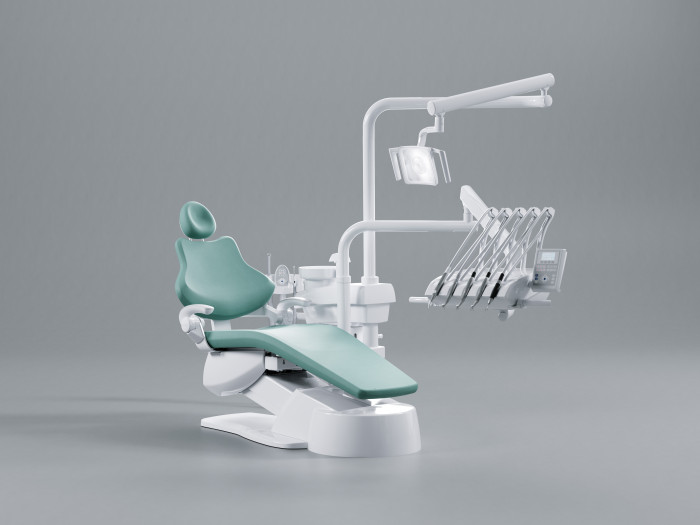
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: KaVo Dental Chairs – Key Considerations for Procurement in 2026
Frequently Asked Questions: Purchasing KaVo Dental Chairs in 2026
| Question | Answer |
|---|---|
| 1. What voltage and electrical requirements are needed for KaVo dental chairs in 2026? | KaVo dental chairs are engineered for global compatibility. Standard models operate on 230V ±10%, 50/60 Hz. For North American markets, dual-voltage configurations (115V/230V) are available upon request. All units comply with IEC 60601-1 safety standards. A dedicated 16A circuit with grounding is recommended. Ensure your clinic’s electrical infrastructure supports surge protection, especially in regions with unstable power grids. |
| 2. Are spare parts for KaVo dental chairs readily available, and what is the supply chain reliability in 2026? | Yes. KaVo maintains an extensive global spare parts network through its parent company, Envista Holdings. In 2026, all critical components—including motors, control panels, upholstery kits, and tubing—are available via regional distribution centers in Europe, North America, Asia-Pacific, and the Middle East. Lead times for standard parts are typically 3–7 business days. Distributors receive priority access to the KaVo eParts Portal for real-time inventory tracking and expedited ordering. Long-term availability of parts is guaranteed for a minimum of 10 years post-discontinuation of any model. |
| 3. What does the installation process for a KaVo dental chair involve, and is professional setup required? | Professional installation by a certified KaVo technician is mandatory to ensure compliance with safety standards and warranty validity. The process includes uncrating, mechanical assembly, electrical and hydraulic connections, integration with dental units (if applicable), and functional testing. On-site calibration of position memory, emergency lowering, and touchscreen interfaces is performed. Average installation time: 3–4 hours per unit. Remote pre-installation site surveys are offered to verify floor load capacity, utility access, and spatial clearance. |
| 4. What is the warranty coverage for KaVo dental chairs purchased in 2026? | KaVo offers a comprehensive 3-year limited warranty on all dental chairs purchased in 2026. This includes coverage for manufacturing defects in materials and workmanship, with extended 5-year warranty options available for critical components (e.g., lift mechanism, base, and control system) through KaVo Care Protection Plans. The warranty is void if installation is not performed by an authorized technician or if non-OEM parts are used. Proof of purchase and installation documentation are required for service claims. |
| 5. How does KaVo support clinics and distributors with post-warranty service and maintenance? | KaVo provides structured post-warranty support via KaVo Service Solutions, including preventive maintenance contracts, remote diagnostics, and rapid-response field service. Certified technicians are available in over 80 countries. Distributors gain access to training programs, technical bulletins, and priority spare parts allocation. All chairs are equipped with Bluetooth-enabled diagnostic modules (from 2024 onward), allowing proactive fault detection and reduced downtime. Service-level agreements (SLAs) guarantee 48-hour response times for critical failures in premium support tiers. |
Need a Quote for Kavo Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


