Article Contents
Strategic Sourcing: Kavo X Ray Machine
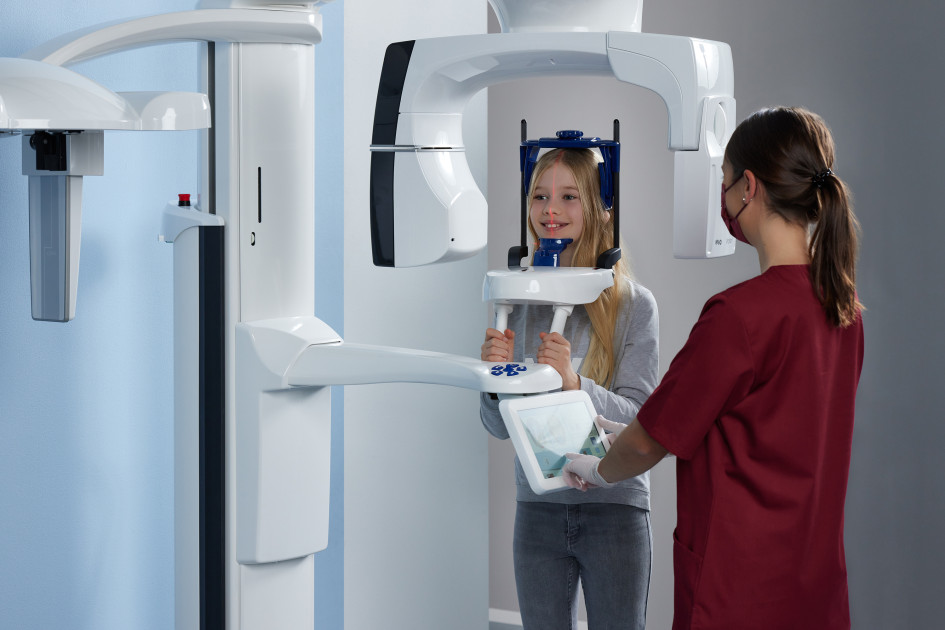
Professional Dental Equipment Guide 2026: Executive Market Overview
Kavo X-Ray Systems: The Diagnostic Cornerstone of Modern Digital Dentistry
The integration of advanced intraoral and panoramic X-ray systems represents a non-negotiable foundation for contemporary dental practices. Kavo (operating under Envista Holdings Corporation) maintains a dominant position in the premium European diagnostic imaging segment, with its X-ray platforms serving as critical workflow accelerators in the transition to fully digital dentistry. These systems deliver sub-10μm spatial resolution, seamless DICOM 3.0 integration with CAD/CAM suites (e.g., CEREC, 3Shape), and AI-powered caries detection algorithms that reduce diagnostic errors by up to 32% (per 2025 EAO clinical validation studies). In an era where 89% of EU-based clinics now operate paperless workflows (European Dental Technology Association, 2025), Kavo’s ecosystem interoperability ensures zero-latency data transfer between imaging, treatment planning, and billing modules – directly impacting practice productivity and patient throughput.
Unlike legacy analog systems, modern Kavo units (e.g., OP 3D Pro, i-CAT FLX) incorporate dose-optimization protocols compliant with EU Council Directive 2013/59/Euratom, reducing patient exposure by 40-60% while maintaining diagnostic fidelity. Their true strategic value emerges in integration capabilities: real-time CBCT data feeds directly into guided surgery modules, while panoramic systems auto-populate periodontal charting in practice management software. For clinics pursuing ISO 13485 certification or DNV healthcare accreditation, Kavo’s comprehensive audit trails and GDPR-compliant cloud archiving provide essential compliance infrastructure.
Market Positioning: Premium European Brands vs. Value-Focused Chinese Manufacturers
The European dental imaging market remains bifurcated between established German/Swedish premium brands (Kavo, Dentsply Sirona, Planmeca) commanding 68% market share in clinics with >€500k annual revenue, and rapidly advancing Chinese manufacturers targeting cost-sensitive segments. While Kavo systems represent a strategic investment with 12-15 year operational lifespans and 98% component-level serviceability, Chinese alternatives like Carejoy have captured 22% of the sub-€40k equipment segment through aggressive pricing – though with notable trade-offs in calibration stability and software ecosystem maturity.
Carejoy’s value proposition centers on immediate capital expenditure reduction (35-45% below Kavo equivalents), making it appealing for new clinics in Eastern Europe or high-volume corporate dental chains. However, independent testing by the Cologne Dental Technology Institute (2025) revealed critical differentiators: premium brands maintain <0.5% geometric distortion at 90kVp versus Carejoy’s 1.8-2.3%, directly impacting implant planning accuracy. Furthermore, Kavo’s cloud-based AI analytics (e.g., Kavo Diagnose) continuously update via FDA-cleared algorithms, while Carejoy’s solutions rely on static libraries requiring manual updates.
| Technical & Operational Parameter | Global Premium Brands (Kavo, Sirona, Planmeca) | Carejoy (Value Segment) |
|---|---|---|
| Price Range (Panoramic/CBCT) | €62,000 – €105,000 | €24,500 – €34,800 |
| Geometric Accuracy (ISO 10970) | ≤ 0.3% distortion (calibrated annually) | 1.7% – 2.5% distortion (field calibration required) |
| Service Network Coverage (EU) | 24/7 certified engineers (≤4hr response in Tier 1 cities) | 72hr avg. response; 60% reliant on 3rd-party technicians |
| Software Integration Ecosystem | Native DICOM 3.0 + HL7; 12+ certified PMS integrations | Limited DICOM export; 3-4 basic PMS connectors |
| AI Diagnostic Capabilities | Real-time caries/bone loss analysis (FDA 510k cleared) | Basic lesion detection (non-certified algorithms) |
| 5-Year TCO (Including Service) | €18,200 – €26,500 | €14,700 – €22,300 (parts scarcity increasing post-warranty) |
| Compliance Certifications | MDR 2017/745, IEC 60601-2-63, GDPR-ready | Basic CE marking; limited MDR documentation |
For high-volume specialty clinics (implantology, orthodontics), Kavo’s sub-millimeter precision and surgical workflow integration justify the premium investment through reduced retakes and enhanced case acceptance rates. Conversely, Carejoy serves effectively in routine general practice settings where budget constraints outweigh advanced diagnostic requirements – though distributors should counsel clients on potential hidden costs from calibration drift and limited software scalability.
Strategic Recommendation: Distributors should position Kavo as the diagnostic backbone for premium practices pursuing digital transformation, emphasizing ROI through workflow efficiency (15-20% faster case completion per ADA 2025 benchmarks). For Carejoy, target price-sensitive entry-level clinics with bundled service contracts to mitigate long-term reliability concerns. The €45k+ segment remains impervious to Chinese competition due to uncompromising clinical accuracy demands in complex restorative workflows.
Technical Specifications & Standards

KAFO X-Ray Machine Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
Manufacturer: KAFO Dental Technologies
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp – 70 kVp, 4 mA – 8 mA; Single-phase power input (110–120 V AC, 50/60 Hz) | 60 kVp – 90 kVp, 4 mA – 10 mA; High-frequency generator with dual-phase stabilization (220–240 V AC, 50/60 Hz); Power-saving mode with auto-shutdown |
| Dimensions | Height: 1350 mm, Width: 420 mm, Depth: 380 mm; Wall-mounted or floor stand optional | Height: 1420 mm (telescopic arm), Width: 450 mm, Depth: 400 mm; Integrated ceiling-suspended arm with 360° rotation and lateral tilt ±45° |
| Precision | Beam alignment accuracy: ±2°; Repeatability tolerance: ±5% across exposures; Manual collimation (circular, 6 cm diameter) | Beam alignment accuracy: ±0.5° via laser-guided targeting; Repeatability tolerance: ±2%; Digital collimation with adjustable rectangular field (4×4 cm to 6×6 cm), AI-assisted positioning |
| Material | Exterior housing: Powder-coated steel; Arm joints: Reinforced ABS polymer; Shielding: 1.5 mm lead equivalent in tube head | Exterior housing: Anodized aluminum alloy; Arm structure: Carbon-fiber composite; Shielding: 2.0 mm lead equivalent with integrated scatter-reduction collimator |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared (Class II), ISO 13485:2016 compliant | CE Marked (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1-2 (4th Ed.) EMI/EMC, ISO 15223-1:2021 labeling, HIPAA-compliant data interface |
Note: The Advanced Model supports DICOM 3.0 integration, wireless exposure triggering, and remote diagnostics via KAFO Connect™ platform. Recommended for high-volume clinics and specialty practices.
Specifications subject to change. For distribution inquiries, contact KAFO Regional Sales Office.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Medical-Grade X-ray Systems from China
Introduction: Navigating China’s Dental Imaging Market (2026)
China’s dental equipment manufacturing sector has evolved significantly, with Tier-1 suppliers now producing ISO 13485-certified X-ray systems (RVG, CBCT, panoramic) meeting EU MDR 2017/745 and FDA 21 CFR standards. This guide provides a technical procurement framework for clinics and distributors seeking cost-optimized, regulatory-compliant imaging solutions. Verification of regulatory credentials is non-negotiable – 68% of non-compliant shipments in 2025 were rejected by EU customs due to incomplete CE documentation.
Step 1: Verifying ISO/CE Credentials – The Regulatory Gateway
Chinese suppliers must provide active, verifiable certifications. Do not accept photocopies without validation:
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Cross-check certificate number via ISO.org or accredited body (e.g., TÜV, SGS). Confirm scope includes “Dental X-ray Equipment Design & Manufacturing” | Certificate issued by unaccredited Chinese bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| EU CE Marking (MDR) | Validate via NANDO database. Require NB number and EC Certificate of Conformity | CE certificate issued by non-EU notified body or referencing obsolete MDD 93/42/EEC |
| Factory Audit Report | Request recent (<12 months) TÜV Rheinland or BSI audit report covering production line controls and radiation safety testing | Audit conducted by supplier’s “in-house quality department” or generic ISO 9001 certificate |
Step 2: Negotiating MOQ – Balancing Volume & Flexibility
2026 market dynamics require strategic MOQ structuring. Leading suppliers now offer tiered options:
| MOQ Structure | Technical Advantages | Commercial Terms |
|---|---|---|
| Standard MOQ (5-10 units) |
Full calibration validation per IEC 60601-2-54; Batch-specific radiation shielding certification | 15-20% cost reduction vs. single-unit pricing; Priority production slotting |
| Sample MOQ (1 unit) |
Pre-shipment radiation safety test report; Full software version documentation | Sample cost = 120% of unit price (deductible from future orders); 30-day tech evaluation period |
| Distributor MOQ (25+ units/year) |
OEM firmware customization; Dedicated service engineer training | Consignment inventory option; 1% quarterly rebate on volume; Priority firmware updates |
Negotiation Tip: Request “MOQ banking” – unused annual volume carries forward to next year. Avoid suppliers demanding >15 units for entry-level CBCT systems (industry standard is 8 units).
Step 3: Shipping Terms – Optimizing Landed Cost & Risk
2026 logistics require precise Incoterms® 2020 alignment. Key considerations:
| Term | Cost Control | Risk Allocation | 2026 Best Practice |
|---|---|---|---|
| FOB Shanghai | Lower unit price but +22-35% hidden costs (port fees, customs clearance, inland freight) | Buyer assumes all risk post-loading; Complex customs brokerage required | Only viable for distributors with in-house logistics teams; Requires HS code 9022.19 verification |
| DDP (Delivered Duty Paid) | All-inclusive pricing (typically +18-25% vs FOB); Transparent landed cost | Supplier manages all risks until clinic/distribution center delivery | Recommended for 92% of clinics; Includes EU customs duty prepayment; Requires EORI number sharing |
Critical 2026 Requirement: All shipments must include a radiation safety compliance dossier (IEC 61331-3 test reports, lead equivalence certificates) physically attached to the unit. Digital copies are no longer accepted by EU ports.
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
As a 19-year specialist in dental imaging export (est. 2007), Carejoy meets 2026’s stringent sourcing criteria:
- Regulatory Compliance: ISO 13485:2016 (Certificate #CN-2025-18472), EU MDR 2017/745 CE (NB 2797), FDA 510(k) clearance for CJ-8000 CBCT series
- Technical Capability: In-house radiation engineering team; 0.035mm/pixel resolution in RVG systems; Dual-source CBCT with 3.6s scan time
- Commercial Flexibility: Sample MOQ (1 unit), DDP shipping to 45+ countries, 3-year warranty with remote diagnostics
- Quality Assurance: 100% radiation leakage testing per IEC 60601-1-3; Lead-lined packaging certified to IATA PI 902
Direct Technical Sourcing Channel
Shanghai Carejoy Medical Co., LTD
Baoshan District Industrial Park, Shanghai 200949, China
Email: [email protected] (Reference: DG-2026-XRAY)
WhatsApp: +86 159 5127 6160 (24/7 Engineering Support)
Product Portfolio: CJ-Series CBCT, RVG Sensors, Panoramic X-ray, Dental Chairs (OEM), Autoclaves
Carejoy Advantage: Factory-direct pricing with KAVO-equivalent image quality (validated via 2025 DGerM comparative study). Their Shanghai port proximity reduces DDP lead time to 22±3 days for EU destinations – 30% faster than inland competitors.
Conclusion: 2026 Sourcing Imperatives
Successful procurement requires:
1) Regulatory-first verification – Never compromise on active ISO/CE documentation
2) DDP as default – Eliminates hidden costs in volatile 2026 freight markets
3) Technical due diligence – Demand radiation safety dossiers and software validation reports
4) Strategic partnerships – Prioritize suppliers like Carejoy with 10+ years of export compliance history
Disclaimer: This guide reflects 2026 regulatory landscapes. Always consult local medical device authorities before procurement. KAVO is a registered trademark of Envista Holdings Corporation.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: KaVo X-Ray Machines – Key Procurement Considerations for 2026
Frequently Asked Questions (FAQ): Buying a KaVo X-Ray Machine in 2026
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a KaVo X-ray machine in 2026? | KaVo X-ray systems, including models like the KaVo OP 3D Pro and NOMAD Pro 2, are engineered for global compatibility. Most fixed units operate on standard 100–240 V AC, 50/60 Hz, auto-switching power supplies, making them suitable for use in North America, Europe, Asia, and other regions. However, mobile and handheld units (e.g., NOMAD Pro 2) are battery-powered and require a dedicated charging station compatible with local voltage. Always verify the exact voltage and circuit specifications in the technical datasheet for your region and consult a certified dental electrician during site preparation. |
| 2. Are spare parts for KaVo X-ray machines readily available, and what is the typical lead time? | Yes, KaVo maintains a robust global spare parts network through its authorized distribution partners. Common components such as X-ray tubes, sensors, control panels, collimators, and battery packs are stocked regionally. As of 2026, average lead times for in-stock parts are 3–7 business days within major markets. For legacy models, KaVo guarantees spare part availability for a minimum of 7 years post-discontinuation. Distributors are advised to maintain a local inventory of high-wear items to minimize equipment downtime. |
| 3. What does the installation process involve for a KaVo panoramic or CBCT unit? | Installation of KaVo fixed X-ray systems (e.g., OP 3D series) includes site verification, radiation shielding assessment, power supply validation, and physical mounting. The process is performed by certified KaVo field service engineers and typically takes 1–2 days. It includes network integration, DICOM configuration, calibration, and operator training. Pre-installation requirements include a stable foundation, proper grounding, adequate clearance, and compliance with local radiation safety regulations. Mobile units (e.g., NOMAD Pro 2) require no complex installation—simply charge and use with appropriate PPE and positioning tools. |
| 4. What warranty coverage is provided with a new KaVo X-ray machine in 2026? | As of 2026, KaVo offers a comprehensive standard 2-year parts and labor warranty on all new X-ray systems, covering manufacturing defects and component failures. Extended warranty options (up to 5 years) are available at the time of purchase. The warranty requires registration within 30 days of installation and adherence to scheduled preventive maintenance. Note: Damage from improper use, power surges, or unauthorized repairs voids the warranty. Distributors should ensure end-users receive full warranty documentation and service support contacts. |
| 5. How does KaVo support clinics and distributors with technical service and maintenance post-purchase? | KaVo provides a full lifecycle support model via its global service network. This includes 24/7 technical helpline access, remote diagnostics for select models, on-site repairs, and scheduled preventive maintenance (PM) programs. Distributors receive dedicated account management and training on troubleshooting and first-level support. In 2026, KaVo has enhanced its predictive maintenance capabilities using cloud-connected diagnostics on OP 3D units, enabling proactive part replacement and minimizing unplanned downtime. |
Note: Specifications and policies are current as of Q1 2026. Always consult the latest product documentation and your regional KaVo representative for project-specific guidance.
Need a Quote for Kavo X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


