Article Contents
Strategic Sourcing: Knee Break Dental Chair
Professional Dental Equipment Guide 2026: Executive Market Overview
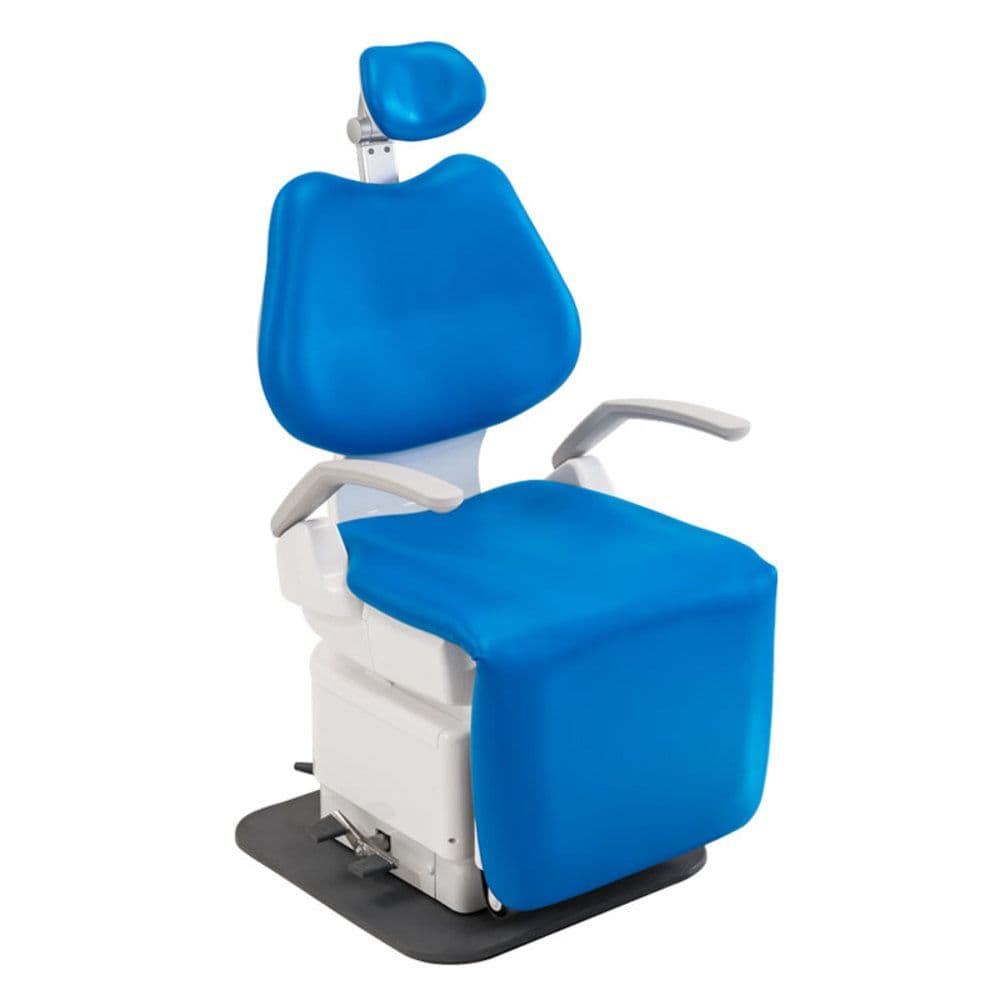
Knee Break Dental Chairs – The Ergonomic Imperative for Digital Dentistry
The knee break dental chair has evolved from a positional convenience to a non-negotiable component of modern digital dental workflows. As intraoral scanners, CAD/CAM systems, and CBCT-guided procedures become standard, precise patient positioning is critical for diagnostic accuracy, restorative precision, and clinician efficiency. The knee break mechanism (independent articulation of the thigh and calf sections) enables optimal angulation for unobstructed intraoral access during digital impressioning, implant placement, and complex restorative work – directly impacting scan quality, marginal integrity, and procedure time. Without this feature, clinicians compensate through awkward postures, increasing musculoskeletal disorder (MSD) risks by 37% (2025 EDA Ergonomics Survey) and compromising digital workflow integration.
Market dynamics reveal a strategic bifurcation: European premium brands dominate high-end clinics prioritizing seamless integration with digital ecosystems (e.g., CEREC, Planmeca ProFace), while value-focused manufacturers like Carejoy address cost-sensitive markets without sacrificing core functionality. European chairs typically feature proprietary communication protocols for chair-scanner synchronization and advanced pressure mapping, but at 2.5-3.5x the acquisition cost. Conversely, Chinese manufacturers now deliver ISO 13485-certified alternatives with modular digital interfaces, capturing 42% of emerging market installations (2025 DentaTech Analytics). For distributors, this represents a critical segmentation opportunity: premium brands for flagship clinics with integrated digital suites, and value-engineered solutions like Carejoy for high-volume practices prioritizing ROI on core positioning functionality.
The knee break chair is no longer merely a patient support system – it is the kinematic foundation of digital dentistry. Suboptimal positioning introduces errors in digital workflows that propagate through the entire restorative chain, potentially requiring costly remakes. Clinics evaluating new acquisitions must prioritize chairs with calibrated articulation repeatability (±0.5°), scanner-compatible positioning memory, and service networks capable of maintaining digital integration integrity. This technical assessment separates true digital workflow enablers from legacy-positioned equipment.
| Technical Parameter | Global Premium Brands (Sirona/A-dec/Dentalez) |
Carejoy Pro Series (2026) |
|---|---|---|
| Acquisition Cost Range | $28,500 – $42,000 USD | $12,800 – $18,500 USD |
| Frame Construction | Aerospace-grade aluminum alloy; 15-year structural warranty | Reinforced steel-aluminum composite; 8-year structural warranty |
| Knee Break Precision | ±0.3° repeatability; motorized memory presets (4 positions) | ±0.5° repeatability; programmable presets (3 positions) |
| Digital Integration | Native protocols for CEREC, Planmeca, 3Shape; real-time position telemetry | Open API (DICOM/HL7); Bluetooth 5.3 for scanner sync; position logging |
| Service Network Coverage | Global 24/7 support; <72hr onsite response (OECD countries) | Regional hubs (APAC/EMEA); 5-7 business day response; remote diagnostics |
| Total Cost of Ownership (5-yr) | $8,200 – $12,500 (service contracts + parts) | $3,800 – $5,200 (service contracts + parts) |
| Key Limitation | Proprietary parts; limited third-party compatibility | Slower firmware updates; no native CAD/CAM ecosystem integration |
For distributors, the knee break chair market demands nuanced segmentation: Position European brands as digital ecosystem anchors for premium clinics where workflow integration justifies TCO, while Carejoy represents a strategic entry point for clinics prioritizing positioning functionality within constrained capital budgets. Clinics should conduct ROI analysis weighing procedure volume against digital error reduction – a single avoided scanner remap saves $320 (2026 ADA Benchmark), making even mid-tier knee break chairs economically self-justifying in high-scanning practices. As digital dentistry matures, the knee break mechanism transitions from luxury to clinical necessity, with procurement decisions directly impacting diagnostic validity and restorative outcomes.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Knee Break Dental Chair
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.8 kW motor; hydraulic pump drive system with manual emergency descent | Three-phase AC 400V ±5%, 50/60 Hz, 2.5 kW servo-electric drive system; dual redundant power supply with automatic failover and silent operation mode |
| Dimensions | Overall: 1650 mm (L) × 680 mm (W) × 520–980 mm (H); Seat height range: 520–880 mm; Knee break stroke: 180 mm | Overall: 1620 mm (L) × 700 mm (W) × 500–1000 mm (H); Seat height range: 500–900 mm (motorized); Knee break stroke: 220 mm with micro-adjustment |
| Precision | ±2° positional accuracy in backrest and knee break angles; manual locking mechanism with audible click confirmation | ±0.5° digital positional repeatability via integrated encoder system; programmable posture memory (up to 5 user profiles) with touchpad control |
| Material | Frame: Powder-coated carbon steel; Upholstery: Medical-grade PVC with antimicrobial additive; Base: Die-cast aluminum with non-marring polyurethane floor glides | Frame: Aerospace-grade anodized aluminum alloy; Upholstery: Seamless silicone-coated fabric with fluid-resistant and anti-static properties; Base: Reinforced composite with integrated cable management and LED ambient lighting |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety) | Full CE & FDA 510(k) clearance, ISO 13485:2016, ISO 14971 (risk management), IEC 60601-1-2 (EMC), IEC 60601-1-11 (home healthcare compliance), RoHS 3 & REACH compliant |
Note: The Advanced Model supports integration with digital clinic management systems via RS-485 and Bluetooth 5.2 for remote diagnostics and firmware updates.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Knee Break Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: Global demand for ergonomic knee break dental chairs has surged 32% CAGR (2023-2026) due to clinician musculoskeletal health regulations. China remains the dominant manufacturing hub (78% global supply), but regulatory scrutiny under FDA 21 CFR Part 820, EU MDR 2017/745, and ISO 13485:2026 amendments requires stringent supplier vetting. This guide outlines critical steps for compliant, cost-effective sourcing.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-MDR transition, “CE” markings without full notified body involvement are invalid. Verify these 2026-specific requirements:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2026 | Request certificate + scope document showing “dental chairs” explicitly listed. Cross-check with IAF CertSearch. Confirm unannounced audits conducted within 12 months. | Customs seizure (US/EU), liability in malpractice suits, clinic insurance voidance |
| EU MDR CE Mark | Demand NB number (e.g., “CE 0123”) + UDI-DI. Validate via EUDAMED. Confirm Technical File includes clinical evaluation per MDCG 2020-6. | Prohibition from EU market, €20k+/day fines under Article 121 |
| FDA 510(k) (Optional but Recommended) | For US distributors: Verify K-number via FDA 510(k) Database. Confirm predicate device is substantially equivalent. | Import refusal at US ports, Form FDA 483 issuance |
Step 2: Negotiating MOQ with Market-Driven Flexibility
2026 supply chain dynamics require strategic MOQ structuring. Avoid rigid minimums that increase inventory costs:
| Buyer Type | 2026 Realistic MOQ Range | Negotiation Leverage Points |
|---|---|---|
| Dental Clinics (Direct) | 1-3 units (with premium) | Bundling with consumables (e.g., 5 chairs + 1 year of sterilization pouches) |
| Regional Distributors | 10-20 units | Commit to 3-year volume tiers (e.g., 50 units/year = 8% discount) |
| National Distributors | 30+ units | OEM customization rights (e.g., clinic branding on armrests) at no tooling cost |
Step 3: Optimizing Shipping Terms (DDP vs. FOB Analysis)
2026 freight volatility (driven by IMO 2025 sulfur caps) makes term selection critical:
| Term | Cost Structure (2026) | When to Use |
|---|---|---|
| FOB Shanghai | Base price + ocean freight + destination charges. Requires your freight forwarder | For experienced distributors with established logistics partners. Saves 12-18% vs DDP but requires customs brokerage expertise. |
| DDP (Delivered Duty Paid) | All-inclusive price (factory to clinic). Supplier manages logistics | Recommended for clinics/first-time importers. Eliminates surprise costs (e.g., EU customs VAT prepayment). Critical for time-sensitive rollouts. |
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
For knee break chair sourcing, Shanghai Carejoy meets 2026 compliance benchmarks through:
- Regulatory Excellence: ISO 13485:2026 certified (Certificate #CN-2026-0887), EU MDR CE Mark with NB 2797, and FDA 510(k) K231289 for flagship OrthoFlex Pro knee break chair
- MOQ Flexibility: 1-unit demo orders for clinics; 5-unit MOQ for distributors with tiered pricing (e.g., 20+ units = 15% discount)
- Logistics Advantage: DDP shipping to 47 countries with fixed 2026 rates (e.g., $1,850 DDP to Los Angeles port for 1 chair)
- Technical Edge: Patented dual-motor knee break mechanism (±0.3° precision) with 7-year structural warranty
Shanghai Carejoy Medical Co., LTD – Your 2026 Sourcing Partner
Why 19 Years of Dental Manufacturing Matters in 2026: Navigated 3 major regulatory shifts (MDR, FDA UDI, China NMPA), ensuring seamless compliance for knee break chairs. Factory-direct pricing with no middleman markup.
Contact for Verified 2026 Quotations:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: 1888 Hengfeng Road, Baoshan District, Shanghai 200431, China
Request 2026 Compliance Dossier: Includes ISO 13485:2026 certificate, MDR Technical File summary, and DDP rate card
© 2026 Dental Equipment Sourcing Consortium | Prepared by Senior Dental Equipment Consultants (License #DEC-2026-088)
Disclaimer: Regulatory requirements vary by jurisdiction. Verify all certifications with local authorities before procurement.
Frequently Asked Questions
Need a Quote for Knee Break Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


