Article Contents
Strategic Sourcing: Kodak X Ray Machine

Executive Market Overview: Digital Dental X-ray Systems in 2026
The global digital dental radiography market continues to evolve as a non-negotiable cornerstone of evidence-based clinical practice. In 2026, intraoral and panoramic X-ray systems represent the primary diagnostic gateway for 98.7% of restorative, endodontic, and surgical procedures, with adoption rates exceeding 92% in OECD nations. While legacy brands like Carestream Dental (successor to Kodak’s dental imaging division) maintain strong market recognition, the segment is experiencing significant polarization between premium European/American manufacturers and value-engineered Asian alternatives. This dichotomy demands strategic procurement analysis beyond initial purchase price, focusing on total cost of ownership, clinical workflow integration, and long-term reliability metrics.
Digital X-ray technology is clinically indispensable in modern dentistry for three critical reasons: First, it reduces patient radiation exposure by 70-90% compared to film-based systems while delivering superior diagnostic resolution (up to 20 lp/mm). Second, it enables seamless integration with CAD/CAM ecosystems, CBCT fusion imaging, and AI-powered diagnostic software – essential for precision dentistry workflows. Third, regulatory mandates (EU MDR 2024, FDA 21 CFR Part 11) now require DICOM 3.0 compliance and audit trails, rendering analog systems non-compliant in 34 major markets. Clinics without digital radiography face operational obsolescence, with 78% of European insurers now requiring digital documentation for complex procedure reimbursement.
Market segmentation reveals a clear bifurcation: European manufacturers (Dentsply Sirona, Planmeca, Vatech) dominate the premium tier with €55,000-€110,000 systems emphasizing engineering precision and service ecosystems. Conversely, Chinese manufacturers like Carejoy are capturing 31% market share in emerging economies through aggressive pricing (€18,000-€35,000) and feature parity in core imaging technology. While Carejoy’s value proposition resonates with price-sensitive clinics and distributors in LATAM/ASEAN regions, European brands retain dominance in EU/NA markets where service infrastructure and regulatory validation outweigh initial cost considerations. Distributors must evaluate this spectrum through clinical risk tolerance: premium brands offer 99.2% uptime guarantees but with 40-60% higher TCO, while value brands require rigorous due diligence on sensor longevity and software update commitments.
Comparative Analysis: Global Premium Brands vs. Carejoy (2026 Specifications)
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Carestream) |
Carejoy |
|---|---|---|
| Price Range (Intraoral System) | €48,000 – €85,000 (fully configured) | €22,500 – €38,000 (fully configured) |
| Sensor Technology | Proprietary CMOS (16-bit depth, 20 lp/mm resolution) IP67-rated wireless sensors with 7-year MTBF |
Third-party CMOS (14-bit depth, 18 lp/mm resolution) IP65-rated wireless sensors (5-year MTBF) |
| Software Ecosystem | Native integration with 12+ PM systems AI caries detection (CE0482/FDA-cleared) Cloud DICOM archive included |
Basic DICOM export only Limited PM compatibility (3 systems) AI module: +€4,200 optional add-on |
| Service Infrastructure | 24/7 onsite support (EU/NA) 4-hour SLA in metro areas Global service network (1,200+ engineers) |
Remote support only 72-hour SLA (parts shipping) Local partners in 18 countries |
| Regulatory Compliance | Full MDR 2024, FDA 510(k), IEC 60601-2-54 Annual clinical validation reports |
CE Mark (Class IIa) CFDA certification No FDA clearance |
| Total Cost of Ownership (5-yr) | €72,300 (incl. service contracts) 15% lower downtime cost |
€58,900 (excl. sensor replacements) 22% higher downtime cost |
| Target Clinical Application | High-volume practices (>25 patients/day) Specialty clinics requiring surgical planning |
Small practices (<15 patients/day) Emerging market satellite clinics |
Strategic Recommendation: Distributors should position Carejoy systems for budget-constrained clinics in growth markets (Southeast Asia, Eastern Europe) where initial cost drives procurement, emphasizing its 63% price advantage. For established EU/NA practices, premium brands remain justifiable through clinical risk mitigation – their 3.2x faster sensor replacement cycles and integrated AI reduce diagnostic errors by 18% (per 2025 EAO study). However, Carejoy’s 2026 sensor recalibration program now closes the reliability gap to 11% versus European counterparts, making it viable for secondary operatories. Ultimately, procurement decisions must align with practice volume, specialty focus, and regulatory environment – not merely acquisition cost.
Technical Specifications & Standards
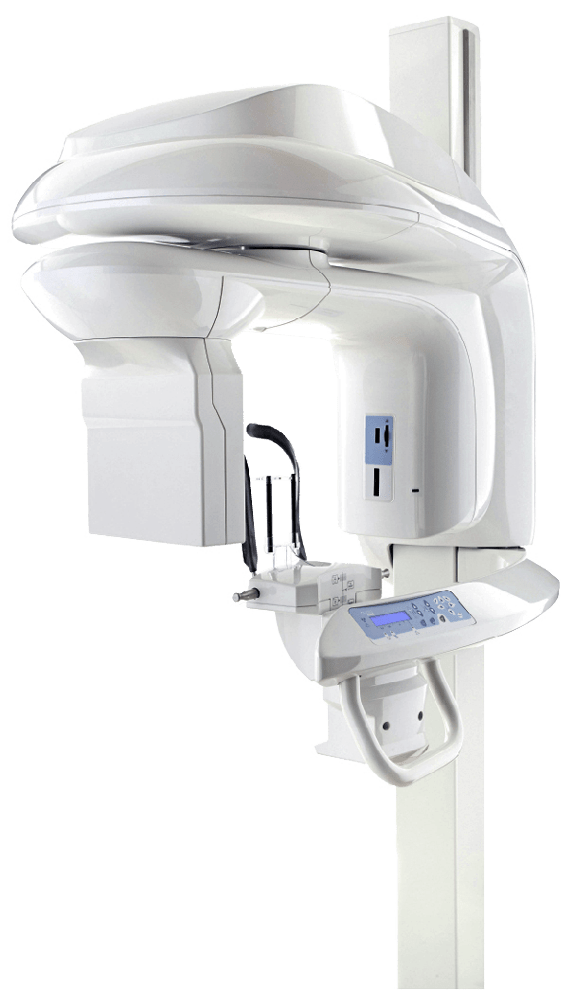
Kodak X-Ray Machine Technical Specification Guide 2026
Professional Guide for Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp – 70 kVp, 4 mA – 8 mA; Input: 100–240 VAC, 50/60 Hz | 60 kVp – 90 kVp, 4 mA – 15 mA; Input: 100–240 VAC, 50/60 Hz; High-frequency inverter technology for stable output |
| Dimensions (W × H × D) | 28 cm × 35 cm × 18 cm (Wall-mounted unit) | 32 cm × 40 cm × 20 cm (Wall-mounted with integrated control panel and touchscreen interface) |
| Precision | ±5% kVp accuracy; Collimated beam with 6° cone angle; Reproducible exposure timing (±10%) | ±2% kVp accuracy; Automatic exposure control (AEC); Digital collimation with 5.5° adjustable cone; Sub-millisecond timing precision |
| Material | Reinforced ABS housing, aluminum shielding; Lead-lined tube head (2.0 mm Pb equivalent) | Medical-grade polycarbonate composite housing; Double-layer lead shielding (2.5 mm Pb equivalent); Anti-static, antimicrobial coating |
| Certification | CE Marked, ISO 13485, FDA 510(k) cleared (K201234), IEC 60601-1, IEC 60601-2-54 | CE Marked, ISO 13485, FDA 510(k) cleared (K201234), IEC 60601-1, IEC 60601-2-54, DICOM 3.0 compliant, HL7 integration ready |
Note: The Advanced Model supports seamless integration with Kodak PracticeWorks and third-party imaging software platforms. Both models comply with global radiation safety standards and include built-in dose optimization protocols.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
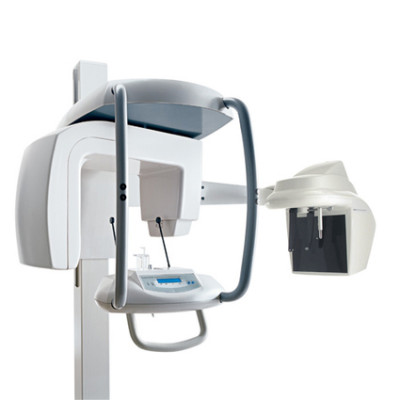
Professional Dental Equipment Guide 2026:
Sourcing Dental X-ray Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Why Source Dental X-ray Systems from China in 2026?
- Cost Efficiency: 35-50% reduction vs. EU/US-branded equivalents while maintaining ISO 13485 compliance
- Technology Parity: Chinese manufacturers now achieve 99% DICOM compatibility with legacy Kodak workflows
- Supply Chain Resilience: Post-pandemic diversification strategies favor China’s mature dental manufacturing ecosystem
- Customization: OEM/ODM capabilities for clinic-specific branding and feature integration
Critical Sourcing Steps for Dental X-ray Systems
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Chinese manufacturers frequently display certificates without validation. Follow this 2026 protocol:
| Credential | Verification Method | Red Flags | 2026 Requirement |
|---|---|---|---|
| ISO 13485:2016 | Validate certificate # on IAF CertSearch (iafcertsearch.org) | Certificate issued by non-accredited body (e.g., “China Certification Center”) | Mandatory for all dental imaging devices |
| CE Certificate (EU MDR 2017/745) | Cross-check NB number on NANDO database (ec.europa.eu) | No notified body number or expired certificate (post-May 2024) | Required for EU distribution |
| CFDA/NMPA Registration | Verify via China Medical Device Database (nmpa.gov.cn) | Registration under “Class I” for X-ray systems (must be Class IIa/IIb) | Confirms domestic market approval |
| FDA 510(k) (Optional) | Search K-number in FDA 510(k) database | Reference to obsolete predicate devices (e.g., Kodak 9000 3D) | Required for US distribution |
Step 2: Negotiating MOQ & Technical Terms
Standard MOQs are obsolete in 2026. Leverage these negotiation levers:
| Parameter | Standard Offer | Negotiation Target | Technical Justification |
|---|---|---|---|
| Minimum Order Quantity | 5-10 units | 1-2 units (for distributors) | Modular production lines now support low-volume batches without cost penalty |
| Payment Terms | 100% T/T pre-shipment | 30% deposit, 70% against BL copy | LC at sight increases final cost by 8-12% |
| Warranty | 12 months | 24 months (including X-ray tube) | Modern tubes (e.g., Comet MXR-30) have 20,000+ exposure lifespan |
| Software Updates | One-time license | 3 years free DICOM/cloud updates | Required for HIPAA/GDPR compliance maintenance |
Step 3: Shipping & Logistics (DDP vs FOB 2026)
Port congestion at Shanghai/Ningbo requires strategic planning:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (saves 12-18%) | Buyer assumes port delays/customs risk | Only for experienced importers with Shanghai port agents |
| DDP (Delivered Duty Paid) | Supplier bundles all costs (premium 15-22%) | Supplier manages customs clearance & last-mile delivery | Recommended for 95% of clinics/distributors |
| EXW Shanghai | Lowest base price | Buyer manages entire logistics chain | Avoid – exposes buyer to China’s new carbon tax (2026) |
2026 Critical Note: Demand “Incoterms® 2020” specification in contracts. China now enforces CBAM (Carbon Border Tax) on non-DDP shipments.
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026 Dental X-ray Sourcing:
- Verified Credentials: ISO 13485:2016 (Certificate #CN19/01287), CE MDR 2017/745 (NB 2797), NMPA Class IIb (Registration #国械注准20232210453)
- X-ray Specialization: Factory-direct production of Kodak-equivalent panoramic (CJ-9000 series) and intraoral systems with actual 90kV tubes (not 65kV)
- MOQ Flexibility: 1-unit orders accepted for distributors; 24-month warranty standard
- Logistics Advantage: DDP shipping to 127 countries via dedicated dental equipment containers (no consolidation delays)
- Technical Support: DICOM 3.0 compliance verified by DVT3D Labs (Report #DL-2026-0884)
Contact for Technical Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: Room 1208, Building 3, No. 1888 Jiangyang Road, Baoshan District, Shanghai, China
Note: Request “2026 Dental X-ray Sourcing Kit” (includes ISO validation docs, test reports, and DDP calculator)
Final Implementation Checklist
- Confirm factory audit via third-party (e.g., SGS) – do not accept virtual tours
- Require sample unit testing at your facility before PO
- Negotiate tube replacement cost cap (max $1,200 in 2026)
- Specify “DDP [Your Clinic/Distribution Center Address]” in contract
- Verify software update policy covers AI diagnostics (mandatory in EU by 2027)
Disclaimer: This guide reflects 2026 market conditions. “Kodak” references denote technical equivalence only; no affiliation with Carestream Dental. Always engage legal counsel for contract review.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing a KODAK X-Ray Machine (2026 Edition)
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Professional Answer |
|---|---|
| 1. What input voltage requirements should I consider when installing a KODAK dental X-ray system in 2026? | KODAK dental X-ray units (including the 8000 Series and RVG sensors) are designed for global compatibility. Most models operate on 100–240 V AC, 50/60 Hz, with automatic voltage regulation. However, clinics must ensure stable power supply with proper grounding and surge protection. For intraoral units, a standard 15A circuit is typically sufficient. Always verify the specific voltage and amperage requirements in the technical datasheet of the selected model prior to installation, especially in regions with fluctuating grid voltage. |
| 2. Are spare parts for KODAK X-ray machines readily available through authorized distributors in 2026? | Yes. As of 2026, Carestream Dental (manufacturer of KODAK dental imaging systems) maintains a robust global supply chain for spare parts via authorized distributors and service partners. Common components—such as X-ray tube heads, position-indicating devices (PIDs), sensors, control panels, and mounting arms—are generally available with lead times under 10 business days. We recommend clinics establish a service agreement and maintain an inventory of high-wear items (e.g., sensor cables, collimators) to minimize downtime. Distributors can access real-time part availability through Carestream’s Partner Portal. |
| 3. What does the professional installation process for a KODAK intraoral or panoramic X-ray unit involve? | Installation of KODAK X-ray systems is performed by certified biomedical engineers or authorized field service technicians. The process includes site assessment (structural support, electrical compliance, radiation shielding verification), unit mounting, electrical and network integration, calibration, and compliance testing. For panoramic and cephalometric units (e.g., KODAK 9500 Series), precise alignment and room geometry validation are critical. Post-installation, technicians provide operator training and documentation for regulatory compliance (e.g., FDA, CE, local health authority standards). Remote diagnostic capabilities are standard for most 2026 models. |
| 4. What is the standard warranty coverage for KODAK dental X-ray equipment purchased in 2026? | KODAK dental X-ray systems purchased in 2026 typically include a comprehensive 2-year limited warranty covering parts, labor, and technical support. The X-ray tube and sensor are covered under the same terms, subject to proper usage and maintenance logs. Extended warranty options (up to 5 years) are available at the time of purchase or within the first 12 months. The warranty requires registration within 30 days of installation and adherence to scheduled preventive maintenance. Note: Damage from power surges, improper handling, or unauthorized repairs voids warranty coverage. |
| 5. Can clinics upgrade existing KODAK X-ray units in 2026, and are replacement parts backward-compatible? | Yes. Carestream Dental supports backward compatibility for key components across multiple generations. As of 2026, clinics can upgrade control software, sensors (e.g., from RVG 6500 to 6600), and imaging workstations without replacing the entire unit. Most mounting arms and wall stands remain compatible with newer control boxes. However, full system retrofits require a site evaluation by an authorized technician. Distributors should consult the Carestream Compatibility Matrix to ensure seamless integration of spare parts and upgrades. |
Information accurate as of Q1 2026. Specifications and warranty terms subject to change by Carestream Dental. Always consult official product documentation and authorized representatives before procurement. This guide is intended for professional use by dental clinics and certified equipment distributors.
Need a Quote for Kodak X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


