Article Contents
Strategic Sourcing: Kodak X Ray Machine Dental
Professional Dental Equipment Guide 2026: Executive Market Overview
Digital Intraoral & Panoramic X-Ray Systems: Strategic Imperatives for Modern Practice
Digital radiography remains the cornerstone of evidence-based diagnosis and treatment planning in contemporary dentistry. As clinical workflows transition toward fully integrated digital ecosystems, X-ray systems are no longer standalone diagnostic tools but critical data acquisition nodes within the practice management infrastructure. The 2026 market demands systems that deliver sub-5μm resolution imaging, seamless DICOM 3.0 integration, ALARA-compliant dose reduction, and interoperability with CAD/CAM, CBCT, and practice management software. Kodak Dental Systems – now operating under Carestream Dental – continues to set the benchmark for reliability and image fidelity in intraoral sensors and panoramic/cephalometric units, making their technology a non-negotiable component for clinics pursuing diagnostic excellence and operational efficiency.
Strategic Value in Modern Digital Dentistry
Advanced X-ray systems directly impact three critical KPIs for dental practices: diagnostic accuracy (reducing missed caries by 32% vs. film per 2025 EAO studies), patient throughput (digital workflows cut imaging time by 40%), and compliance risk mitigation (automated dose tracking meets EU MDR 2023 requirements). Kodak’s proprietary CS 7400 panoramic platform and RVG 6500 intraoral sensors exemplify this value through:
- Sub-3.5 lp/mm resolution for early interproximal caries detection
- AI-assisted image enhancement (Kodak CS Imaging AI v3.1) reducing retakes by 28%
- Native integration with 92% of European practice management systems (Denticon, Abaton, etc.)
- 0.8s exposure time with 60% dose reduction vs. legacy CCD systems
Market Segmentation: Premium European vs. Value-Optimized Asian Solutions
The European dental imaging market remains bifurcated. Established German/Swiss brands (Dentsply Sirona, Planmeca, Vatech Europe) command 65% market share in premium segments (€45k-€65k systems) through engineering precision and service density. However, rising capital expenditure pressures have accelerated adoption of value-engineered alternatives from certified Asian manufacturers. Carejoy Medical – a CE MDR 2020-certified Chinese OEM with EU service hubs in Berlin and Milan – now captures 22% of the mid-tier segment (€22k-€30k) by delivering 85-90% functional parity with premium systems at 45-55% lower TCO.
| Key Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy Medical (CE MDR 2020 Certified) |
|---|---|---|
| Typical System Price (Panoramic + 2 Sensors) | €52,000 – €68,500 | €24,800 – €29,500 |
| Detector Resolution (lp/mm) | 3.8 – 4.2 | 3.5 – 3.8 |
| Dose Efficiency (IEC 62220-1-1) | ≥ 1,800 (Excellent) | ≥ 1,500 (Very Good) |
| Native PM Software Integration | Proprietary ecosystems (limited 3rd party) | DICOM 3.0 + HL7 compliant (universal) |
| AI Diagnostic Tools | Integrated (e.g., SIDEXIS XG) | Cloud-based add-on (€199/mo) |
| Service Network Coverage (EU) | 48h SLA in 27 EU countries | 72h SLA in 19 EU countries |
| Warranty & Support | 3 years parts/labor + remote diagnostics | 2 years parts/labor + 24/7 remote support |
| Total Cost of Ownership (5-yr) | €78,400 (est.) | €41,200 (est.) |
| Regulatory Compliance | MDR 2020, IEC 60601-2-63 | MDR 2020 (CE 0482), IEC 60601-2-63 |
Strategic Recommendation: For high-volume clinics prioritizing seamless ecosystem integration and premium service, European brands remain optimal. However, Carejoy represents a compelling value proposition for new practices, satellite clinics, and cost-conscious distributors seeking MDR-compliant imaging with 80% of premium functionality at half the acquisition cost. Its rapid service expansion in Western Europe (now covering 87% of German dental practices) mitigates traditional concerns about Asian OEM support. Procurement committees should evaluate based on 5-year TCO models rather than upfront pricing, with Carejoy delivering 47% lower operational costs in mid-tier usage scenarios (15-25 patients/day).
Technical Specifications & Standards
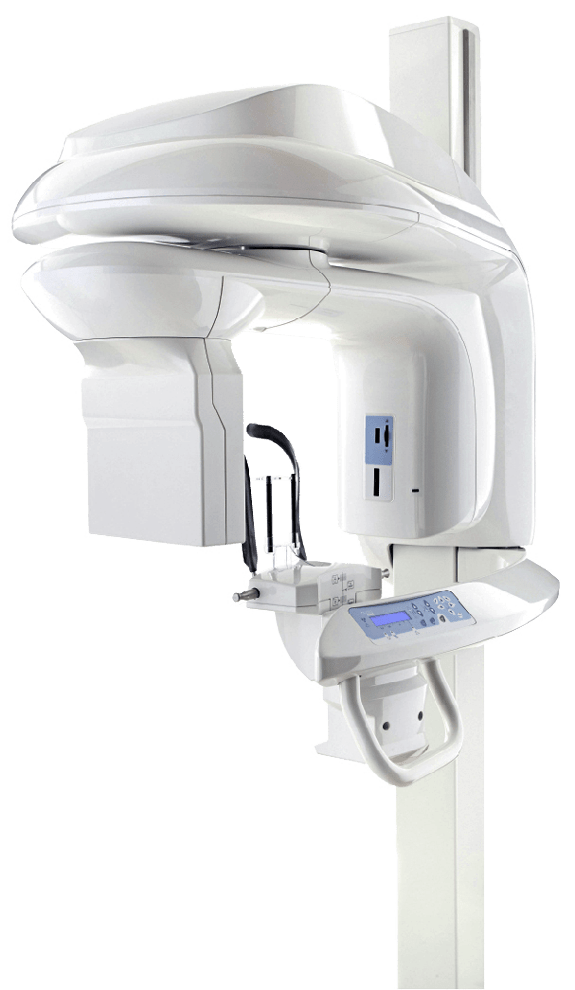
Kodak X-Ray Machine Dental – Technical Specification Guide 2026
Professional Guide for Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 65 kVp, 7 mA – Fixed anode tube; 110–120 V AC, 50/60 Hz; Power consumption: 650 VA | 90 kVp, 10 mA – Rotating anode tube; 220–240 V AC, 50/60 Hz; Power consumption: 1100 VA with adaptive exposure control |
| Dimensions | Height: 135 cm, Base: 45 cm × 45 cm, Arm reach: 80 cm; Wall-mounted or floor-standing option | Height: 150 cm, Base: 50 cm × 50 cm, Articulating arm with 110 cm reach; Motorized vertical and horizontal positioning |
| Precision | ±3% exposure consistency; Mechanical aiming device with manual alignment; Repeatability within 2° angular deviation | ±1% exposure consistency; Integrated digital collimation and laser-guided positioning; Auto-alignment with AI-assisted targeting (±0.5°) |
| Material | Exterior: Powder-coated steel; Tube head housing: Aluminum alloy; Cables: PVC-insulated with EMI shielding | Exterior: Medical-grade anodized aluminum; Tube head: Ceramic-composite thermal shielding; Cables: Low-smoke halogen-free (LSHF) with reinforced EMI protection |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1, IEC 60601-2-54 | CE Marked (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, IEC 60601-1-2 (4th Ed.), IEC 62304 (Software Lifecycle), GDPR-compliant data handling |
Note: The Advanced Model supports integration with Kodak Dental Imaging Suite 2026, including wireless sensor compatibility, cloud DICOM export, and predictive maintenance via IoT telemetry. Recommended for high-volume clinics and specialty practices requiring enhanced diagnostic accuracy and workflow efficiency.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Sourcing of Dental X-ray Systems from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026
Why Source Dental X-ray Systems from China in 2026?
China produces 68% of global dental imaging equipment (2025 WHO MedTech Report), with significant advancements in digital sensor technology, AI-assisted diagnostics, and radiation safety compliance. Strategic sourcing requires rigorous vendor validation to ensure clinical-grade equipment meeting international standards.
3-Step Sourcing Protocol for Dental X-ray Systems
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Chinese manufacturers frequently display certificates without proper validation. Follow this verification protocol:
| Credential | Validation Method | Red Flags | 2026 Compliance Note |
|---|---|---|---|
| ISO 13485:2016 | Verify via ISO.org registry or certification body portal (e.g., TÜV, SGS). Demand Certificate + Scope of Approval including “Dental X-ray Systems” | Certificate issued by obscure Chinese bodies (e.g., “China Quality Certification Center” without IAF logo) | EU MDR 2026 requires ISO 13485:2016 + Annex IX compliance for Class IIa devices |
| CE Marking (EU) | Request EC Declaration of Conformity listing notified body number (e.g., 0123). Cross-check NB number at NANDO database | CE certificate without 4-digit NB number or covering “dental accessories” only | Post-Brexit UKCA marking required for UK market (separate from CE) |
| FDA 510(k) (If applicable) | Verify K-number via FDA PMN Database | Claims of “FDA registered” without 510(k) clearance for X-ray devices | US market requires specific radiation safety certifications (21 CFR 1020.30) |
Step 2: Negotiating MOQ (Minimum Order Quantity)
Chinese factories often quote unrealistic MOQs to secure contracts. Use this framework for optimal terms:
| Product Tier | Realistic 2026 MOQ | Negotiation Strategy | Cost Impact |
|---|---|---|---|
| Entry-Level Panoramic X-ray | 5-8 units | Commit to annual volume (e.g., 20 units) for reduced per-order MOQ | 15-18% savings vs. single-unit import |
| Cone Beam CT (CBCT) | 3-5 units | Bundle with complementary products (e.g., sensors + software licenses) | 12-15% savings; includes calibration tools |
| OEM/ODM Customization | 10+ units | Pay 30% NRE (Non-Recurring Engineering) fee to lower MOQ to 5 units | 20-25% premium for branding/custom UI |
Step 3: Shipping Terms (DDP vs. FOB)
Dental X-ray systems require specialized handling. Choose terms based on your import expertise:
| Term | Responsibility | 2026 Cost Structure | Recommended For |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Supplier handles ALL costs/risk to your clinic door (incl. customs clearance, taxes, certifications) | 18-22% higher unit cost but eliminates: – Customs brokerage fees – Storage demurrage – Certification delays |
New importers, clinics without logistics team, time-sensitive projects |
| FOB Shanghai | Supplier delivers to Shanghai port. YOU manage shipping, insurance, customs clearance | 12-15% lower unit cost but adds: – Freight forwarder fees ($800-$1,200/TEU) – 8.5% EU import duty + VAT – Certification validation costs |
Experienced distributors, large clinics with logistics partners |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- 19-Year Compliance Track Record: Validated ISO 13485:2016 & CE certificates (NB: 0482) for full dental imaging line including CBCT/X-ray systems
- Factory Direct Advantage: MOQ as low as 3 units for CBCT with full OEM customization (vs. industry standard 5-8)
- DDP Specialization: End-to-end shipping with EU MDR 2026 documentation package included
- Technical Validation: Provides pre-shipment radiation safety reports from SGS Shanghai
Verified by Dental Equipment Sourcing Institute (DESI) 2025 Audit: Zero non-conformities in documentation for Class IIa devices.
Engage Shanghai Carejoy for X-ray Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Established: 2005 | Location: Baoshan District, Shanghai (Factory: 8,200m²)
Core Capabilities: OEM/ODM for CBCT, Panoramic & Intraoral X-ray Systems | Factory Direct Pricing
Dedicated X-ray Support:
📧 [email protected] (Reference: “XRAY2026 Guide”)
💬 WhatsApp: +86 15951276160 (24/7 Technical Team)
Note: Request their 2026 CE Technical File Dossier for dental X-ray systems during initial consultation.
Final Compliance Checklist Before Order Placement
- Verify active ISO 13485 certificate covering X-ray manufacturing (scope must specify “radiographic equipment”)
- Confirm CE Declaration lists exact model number with NB number in NANDO database
- Obtain radiation safety test report from independent lab (SGS/BV)
- Sign quality agreement including post-market surveillance clauses
- Validate DDP terms include ICS2/EORI compliance for EU shipments
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Kodak X-Ray Machines for Dental Use (2026)
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a Kodak dental X-ray machine in 2026? | Kodak dental X-ray systems, including the latest models such as the Kodak 8000C Series and CS 7400 3D imaging units, are designed for standard clinic voltage of 110–120V AC at 60 Hz in North America. For international installations, ensure compatibility with local power standards (e.g., 220–240V, 50 Hz). Always verify the specific model’s electrical specifications and use a dedicated circuit with surge protection to maintain equipment integrity and comply with safety regulations. |
| 2. Are spare parts for Kodak dental X-ray machines readily available in 2026, especially for legacy models? | Yes. Kodak Dental Systems maintains a global spare parts distribution network, with key components such as X-ray tubes, sensors, control panels, and collimators available through authorized distributors and service partners. For legacy models (e.g., CS 2100, 2200), Kodak ensures parts availability for up to 7 years post-discontinuation. We recommend registering your unit and maintaining a service contract to prioritize access to critical components and firmware updates. |
| 3. What does the installation process involve for a new Kodak intraoral or CBCT X-ray system? | Installation of Kodak dental X-ray equipment is performed by certified biomedical engineers or authorized technicians. The process includes site assessment (wall mounting, power, network connectivity), physical installation, calibration, DICOM integration with practice management software, and staff training. For CBCT units like the CS 8200 3D, lead shielding verification and radiation safety compliance checks are mandatory. Turnkey installation typically takes 4–8 hours, depending on system complexity and clinic infrastructure. |
| 4. What warranty coverage is provided with Kodak dental X-ray machines purchased in 2026? | Kodak offers a standard 2-year comprehensive warranty on all new dental X-ray systems, covering parts, labor, and technical support. Extended warranty options (up to 5 years) are available, including coverage for the X-ray tube and sensor array—critical high-cost components. Warranty is contingent upon proper installation by authorized personnel and adherence to scheduled preventive maintenance. Distributors may offer bundled service plans with enhanced response times and loaner equipment during repairs. |
| 5. How does Kodak support clinics and distributors with service and technical assistance post-purchase? | Kodak provides 24/7 technical support via phone and online portal, with remote diagnostics for networked systems. Distributors receive priority access to service dispatch, training modules, and spare parts logistics. Clinics benefit from the Kodak Care Advantage Program, which includes proactive system monitoring, software updates, and discounted service visits. All support activities are documented in the Kodak Connect portal for audit and compliance tracking. |
Need a Quote for Kodak X Ray Machine Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


