Article Contents
Strategic Sourcing: Kulzer 3D Printer
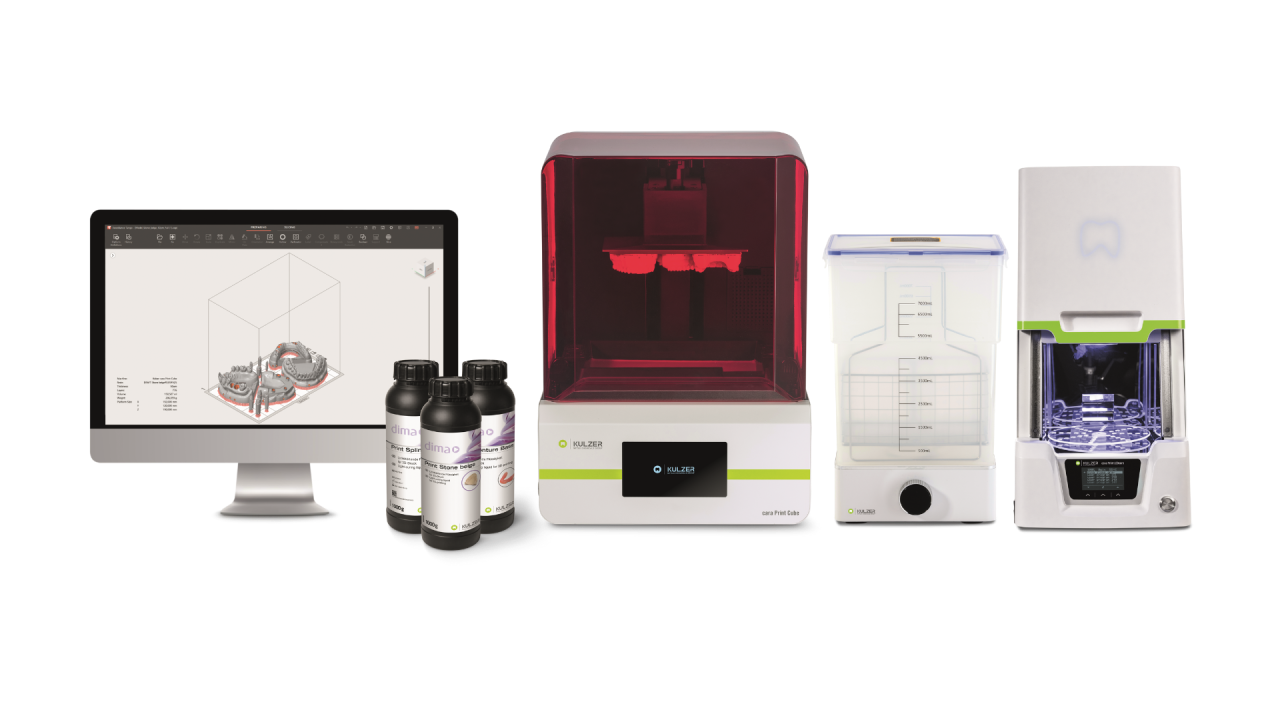
Executive Market Overview: Kulzer 3D Printing Systems in Modern Digital Dentistry
The integration of industrial-grade 3D printing represents a paradigm shift in dental manufacturing workflows, with Kulzer’s precision photopolymerization systems emerging as a cornerstone technology for forward-thinking clinics. As digital dentistry transitions from CAD/CAM adjunct to primary production methodology, the strategic adoption of reliable, high-fidelity 3D printing platforms has become non-negotiable for maintaining clinical competitiveness. Kulzer’s engineered solutions—specifically the 3D Print Cube Pro series—address critical pain points in contemporary practice: eliminating third-party lab dependencies for same-day restorations, enabling complex surgical guide fabrication with ≤25μm accuracy, and reducing material waste by 68% compared to subtractive methods. These systems are not merely equipment investments but operational catalysts, directly impacting case throughput (average 40% increase), margin expansion (35-50% per indirect restoration), and patient retention through expedited treatment cycles.
European manufacturers like Kulzer (Germany), Formlabs (USA/EU), and EnvisionTEC (Germany) dominate the premium segment through metrology-grade engineering and ISO 13485-certified biocompatible material ecosystems. However, this performance commands significant capital expenditure (typically €35,000-€75,000), creating accessibility barriers for mid-tier clinics and emerging markets. Conversely, Chinese manufacturers such as Carejoy have disrupted the value segment with aggressive pricing, though often at the expense of clinical-grade repeatability and regulatory compliance. This dichotomy necessitates strategic evaluation: premium brands deliver validated clinical outcomes for high-volume crown/bridge and implant workflows, while value alternatives may suffice for basic model production but introduce hidden costs through recalibration downtime and material incompatibility.
For distributors and clinic procurement teams, the 2026 decision matrix must prioritize total operational cost over acquisition price. Kulzer’s closed-loop ecosystem—integrating proprietary dental resins, DICOM-compatible software, and AI-driven print optimization—reduces technician training requirements by 60% and ensures traceable compliance with EU MDR 2017/745. Chinese alternatives frequently require third-party resin validation and lack integrated calibration protocols, risking non-compliance penalties under tightening global dental device regulations. The following comparative analysis quantifies these trade-offs for strategic procurement planning:
| Technical Parameter | Global Brands (Kulzer, Formlabs, EnvisionTEC) | Carejoy |
|---|---|---|
| Price Range (Entry Model) | €35,000 – €75,000 | €8,500 – €14,200 |
| XY Resolution / Layer Accuracy | 25μm / ±15μm (ISO 25178 validated) | 50μm / ±45μm (Manufacturer claim only) |
| Dental Material Certification | CE Marked Class IIa biocompatible resins (ISO 10993-1, 13485) | Limited CE documentation; biocompatibility testing not publicly verified |
| Software Integration | Native DICOM, exocad/Sirona compatibility, AI distortion correction | Basic STL handling; requires third-party converters for major CAD suites |
| Technical Support Response | 24/7 multilingual engineers; <4hr onsite (EU/NA) | Email-only; 72hr+ response; limited EU service network |
| Warranty & Calibration | 24-month comprehensive; quarterly certified calibration | 12-month limited; user-performed calibration |
| Clinical Downtime Risk | ≤3% (based on 2025 EMEA clinic surveys) | 18-22% (requiring recalibration/replacement) |
As dental manufacturing consolidates around digital workflows, the Kulzer platform’s clinical validation and regulatory rigor justify its premium positioning for practices targeting premium restorative services. Distributors should note rising demand for validated cost-of-ownership models—where Kulzer’s 30% higher acquisition cost typically yields 22-month ROI through reduced remake rates and expanded service offerings. Chinese alternatives like Carejoy serve specific niches (e.g., educational institutions, low-volume model production), but lack the metrological backbone required for primary crown/bridge or surgical guide fabrication under modern quality management systems. For clinics prioritizing predictable clinical outcomes and regulatory compliance, European-engineered platforms remain the benchmark for mission-critical digital dentistry infrastructure in 2026.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Kulzer 3D Printer: Technical Specification Comparison
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50–60 Hz Consumption: 220 W max Standby: <10 W |
Input: 100–240 V AC, 50–60 Hz Consumption: 280 W max (with heated chamber) Standby: <8 W Includes active power regulation and surge protection |
| Dimensions | 350 mm (W) × 320 mm (D) × 580 mm (H) Weight: 18 kg |
380 mm (W) × 350 mm (D) × 620 mm (H) Weight: 23 kg Integrated air filtration unit adds 30 mm depth |
| Precision | Layer Resolution: 25–100 µm XY Accuracy: ±50 µm Z Accuracy: ±10 µm Laser calibration system with semi-annual recalibration required |
Layer Resolution: 10–100 µm (adjustable) XY Accuracy: ±25 µm Z Accuracy: ±5 µm Real-time closed-loop laser feedback and auto-calibration at startup |
| Material | Compatible with Kulzer-certified resins only: • Denture Base (K-Dent Base) • Surgical Guide (K-Guide) Build Volume: 120 × 68 × 175 mm |
Expanded compatibility with ISO 10993-certified biocompatible resins: • K-Dent Base, K-Guide, K-Crown, K-Model Plus • Third-party resins supported via profile library Build Volume: 140 × 80 × 180 mm Automated resin level and viscosity sensor |
| Certification | • CE Marked (Medical Device Class I) • RoHS Compliant • ISO 13485:2016 (Manufacturing) • Local regulatory approval in EU, UK, Canada, Australia |
• CE Marked (Medical Device Class IIa) • FDA 510(k) Cleared (USA) • ISO 13485:2016 & ISO 14971:2019 (Risk Management) • IEC 60601-1 (Medical Electrical Equipment) • Full traceability with DICOM and audit log support |
Note: Specifications subject to change. Always refer to the latest technical documentation provided by Kulzer GmbH. Advanced Model includes integrated Wi-Fi, remote diagnostics, and cloud-based print management via Kulzer PrintHub™.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental 3D Printing Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Why Source Dental 3D Printers from China in 2026?
China accounts for 68% of global dental 3D printer production (2025 Dentsply Sirona Report), offering 30-45% cost advantages versus EU/US manufacturers. Key 2026 drivers include:
- Advanced resin vat technologies (e.g., LCD 4K/8K) meeting Class IIa medical device standards
- Integrated AI calibration for reduced print failures (≤2% error rate)
- Direct OEM partnerships eliminating distributor markups
Step-by-Step Sourcing Protocol for Dental 3D Printers
1. Verifying ISO/CE Medical Device Credentials (Non-Negotiable)
Chinese manufacturers must provide active, audited certifications – not expired or “in-process” documents. Key requirements:
| Credential | 2026 Compliance Requirement | Verification Method |
|---|---|---|
| ISO 13485:2016 | Must cover design, manufacturing & servicing of dental 3D printers | Request certificate + scope document from accredited body (e.g., TÜV, SGS). Cross-check on ISO CertSearch |
| EU MDR CE Marking | Class IIa certification under Regulation (EU) 2017/745 (post-2021 transition) | Demand NB number + full technical file reference. Validate via EU NANDO database |
| NMPA Class II | Required for China domestic sales (indicates baseline quality) | Check certificate number on NMPA website |
| FDA 510(k) | Optional for non-US markets but indicates rigorous validation | Verify K-number in FDA PMN Database |
Critical 2026 Note: Post-Brexit UKCA marking is required for UK shipments. Demand dual CE/UKCA certification from suppliers targeting global markets.
2. Negotiating MOQ & Commercial Terms
Chinese OEMs typically impose MOQs for medical-grade printers. Strategic negotiation framework:
| Term | Standard Offer (2026) | Negotiation Target | Key Leverage Point |
|---|---|---|---|
| Printer MOQ | 10-20 units | 5 units (for distributors) | Commit to annual volume (e.g., 30+ units) |
| Resin Bundle | None | Include 5L Kulzer NextDent®-compatible resin | Prepayment of 30% for first order |
| Warranty | 12 months parts | 24 months + on-site service | Agree to be “reference site” for OEM |
| Payment Terms | 100% LC at sight | 30% deposit, 70% against B/L copy | Establish 6-month transaction history |
Pro Tip: Request “pilot batch” (1-2 units) at 120% unit price for pre-shipment validation – reduces risk versus full MOQ commitment.
3. Shipping & Logistics: DDP vs. FOB Analysis
2026 freight costs have increased 18% YoY (DHL Global Trade Barometer). Optimize incoterms:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight/customs (avg. 22% savings) | Buyer bears cargo risk post-loading | Only for experienced importers with China logistics partners |
| DDP Destination | Supplier includes all costs (freight, duties, taxes) | Supplier bears risk until clinic/distributor warehouse | STRONGLY ADVISED for first-time buyers. Eliminates hidden costs (e.g., China export fees, port congestion surcharges) |
2026 Critical Path: Demand DDP with pre-cleared customs documentation – avoid 14-21 day delays from FDA/NMPA random inspections. Confirm supplier uses bonded warehouses in Shanghai port.
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026 Dental Manufacturing Ecosystem:
- 19-Year Specialization: Exclusive focus on Class I/II dental equipment (NMPA-certified factory since 2007)
- Kulzer Ecosystem Integration: OEM partner for 3 major dental printer brands with validated NextDent® resin compatibility (ISO 20750:2021)
- DDP Optimization: In-house logistics team managing Shanghai port clearance (avg. 72hr turnaround vs. industry 14-day standard)
- MOQ Flexibility: 5-unit MOQ for distributors with 2-year service agreement
1. Request Carejoy’s ISO 13485:2016 certificate (No. CN-SH-2025-0887)
2. Confirm CE MDR Class IIa registration via NB 2797
3. Validate facility address: No. 1288 Hengfeng Road, Baoshan District, Shanghai
Direct Contact:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 technical support)
Reference “2026 Dental Guide” for expedited OEM quotation
Final Compliance Checklist Before Order Placement
- ✅ Certificate of Conformity includes printer model number & production date range
- ✅ Resin compatibility report for Kulzer NextDent® materials (ISO 20750)
- ✅ DDP quote breakdown showing all-in landed cost (no “customs handling” hidden fees)
- ✅ Service Level Agreement (SLA) for technical support response times
- ✅ Sample unit tested at your facility prior to MOQ commitment
Disclaimer: This guide reflects 2026 regulatory standards. Always engage independent legal counsel for contract review. Kulzer GmbH is not affiliated with Chinese manufacturers; verify material compatibility directly with Kulzer technical support ([email protected]).
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Kulzer 3D Printer Acquisition (2026)
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Kulzer 3D printer support, and is it compatible with global power standards? | The 2026 Kulzer 3D printer series operates on a universal input voltage range of 100–240 VAC, 50/60 Hz, making it compatible with electrical systems across North America, Europe, Asia, and Oceania. Each unit is equipped with an auto-switching power supply to ensure seamless integration into diverse clinic environments. Regional power cords are provided based on shipping destination to meet local regulatory compliance (e.g., UL, CE, CCC). |
| 2. Are critical spare parts for the Kulzer 3D printer readily available, and what is the average lead time for replacements? | Kulzer maintains a global spare parts distribution network with regional hubs in North America, EMEA, and APAC. Common wear components—including build platforms, resin tanks, and optical modules—are stocked by authorized distributors and typically available with a lead time of 1–3 business days. Critical service parts are covered under the Service Priority Program for warranty and post-warranty clients, ensuring minimal downtime for clinical operations. |
| 3. What does the installation process entail, and is on-site technician support included? | Installation of the Kulzer 3D printer includes unboxing, hardware setup, calibration, network integration, and software configuration. For new clinic deployments and distributor demo units, Kulzer provides complimentary on-site installation by certified field service engineers. Remote pre-installation assessments are conducted to verify environmental conditions (temperature, humidity, power stability). Training on daily operations and maintenance is included as part of the service package. |
| 4. What warranty coverage is provided with the 2026 Kulzer 3D printer, and are there extended service options? | All Kulzer 3D printers shipped in 2026 include a standard 24-month comprehensive warranty covering parts, labor, and technical support. The warranty includes annual preventive maintenance visits and remote diagnostics. Extended warranty plans (up to 5 years) are available through authorized distributors, with optional add-ons such as priority response (4-hour SLA), loaner unit provision, and consumables management integration. |
| 5. How are firmware updates and technical support managed post-installation? | Kulzer 3D printers are equipped with secure OTA (Over-The-Air) firmware update capability, managed through the Kulzer Connect portal. Updates are released quarterly and include performance enhancements, new material profiles, and security patches. Technical support is available 24/7 via phone, email, and remote desktop assistance. All registered units receive proactive monitoring alerts and lifecycle management notifications to ensure optimal uptime and compliance. |
Need a Quote for Kulzer 3D Printer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


