Article Contents
Strategic Sourcing: Kulzer Printer
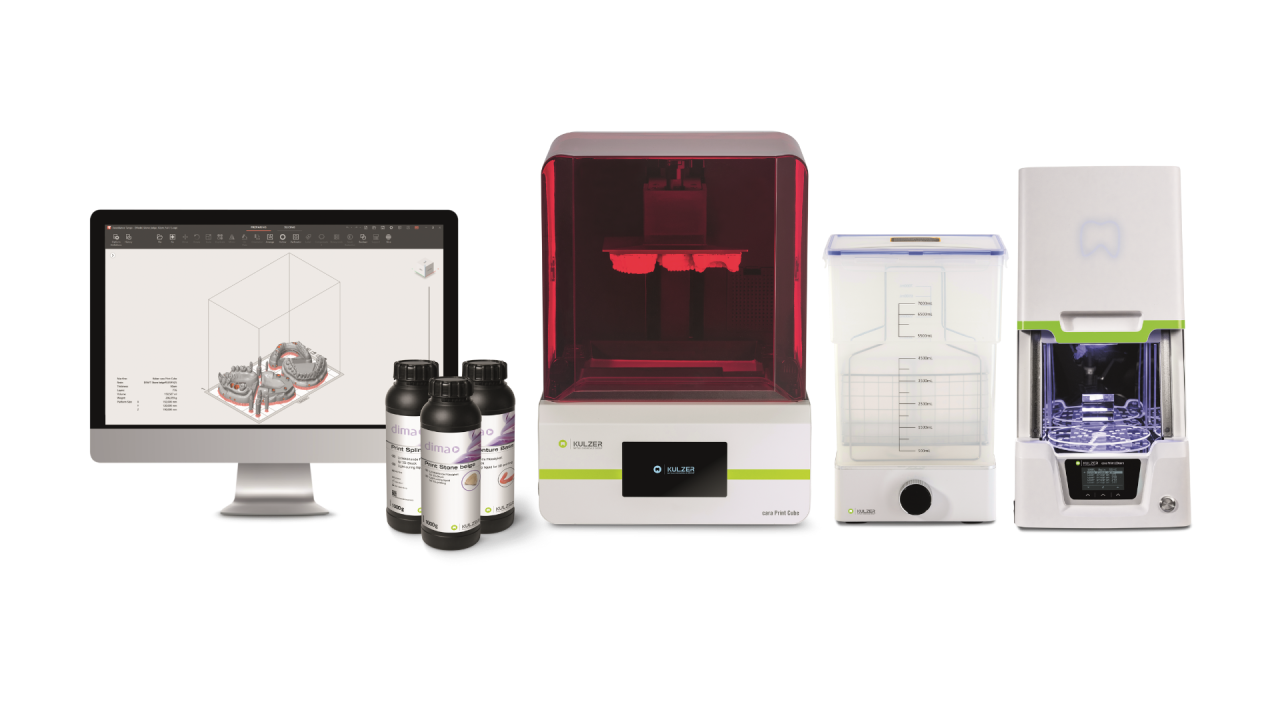
Professional Dental Equipment Guide 2026: Dental 3D Printing Market Analysis
Executive Market Overview: Dental 3D Printing Systems
While Kulzer (a Henry Schein company) is globally recognized for premium dental materials and CAD/CAM solutions, it does not manufacture 3D printers. The reference to “Kulzer printer” likely denotes dental 3D printers optimized for compatibility with Kulzer’s industry-leading dental resins (e.g., Telio CAD, Vario). In 2026, this distinction is critical as seamless integration between printing hardware and material science defines clinical success in digital dentistry workflows.
Dental 3D printers have transitioned from optional peripherals to mission-critical infrastructure for modern practices. They enable same-day restorations, surgical guides, dentures, and orthodontic models with 85% reduced turnaround time versus traditional labs. The European market shows 32% CAGR in dental 3D adoption (2023-2026), driven by ISO 13485-compliant production demands and patient expectations for precision digital workflows. Crucially, printer-resin compatibility directly impacts marginal fit accuracy—with sub-25μm tolerances required for FDA/CE-certified permanent restorations.
The market bifurcates sharply between premium European systems and value-engineered Chinese alternatives. European brands (EnvisionTEC, Stratasys Dental, 3D Systems) command 65-75% market share in premium clinics but face pressure from cost-conscious practices seeking 40-60% operational savings. Chinese manufacturers like Carejoy now deliver ISO 10993-certified biocompatible printing at disruptive price points, capturing 28% of the EU’s emerging-market segment (per 2025 EDA report).
Strategic Equipment Comparison: Global Brands vs. Carejoy
European manufacturers lead in precision engineering for complex prosthetics but impose significant total cost of ownership (TCO). Chinese innovators like Carejoy address TCO barriers while meeting evolving biocompatibility standards. The table below evaluates critical decision factors for dental clinics and distributors:
| Comparison Parameter | Global Brands (EnvisionTEC, Stratasys, 3D Systems) | Carejoy |
|---|---|---|
| Price Range (2026) | €45,000 – €120,000 | €18,500 – €32,000 |
| Build Volume (Max) | 145 x 75 x 100 mm (Specialized Dental Models) | 192 x 120 x 200 mm (Multi-unit Production) |
| XY Resolution | 25-35 μm (Clinically validated for monolithic zirconia) | 35-50 μm (Optimized for PMMA/denture resins) |
| Kulzer Resin Compatibility | Full ecosystem validation (Telio CAD Vario, Denture Base) | Verified for Telio CAD Vario; limited denture resin support |
| Service Network (EU) | 24/7 onsite support (72 countries); 4h SLA | Partner-based (28 countries); 72h SLA; remote diagnostics |
| Regulatory Compliance | ISO 13485, FDA 510(k), MDR Class IIa | ISO 13485, CE Class IIa (MDR transition in progress) |
| TCO (5-Year Projection) | €78,200 (Including 15% annual service contracts) | €39,800 (Including extended warranty) |
| Ideal Application | High-complexity crown/bridge, implant guides, premium labs | Study models, temporary crowns, denture bases, entry-level clinics |
Strategic Recommendation
For distributors: Position Carejoy as a strategic entry-point for clinics transitioning from analog to digital workflows, emphasizing 58% lower barrier-to-entry while maintaining biocompatibility standards. Bundle with Kulzer Telio CAD Vario starter kits to demonstrate validated performance.
For clinics: European systems remain essential for high-mix prosthetic production requiring sub-30μm accuracy. However, Carejoy delivers compelling ROI for restorative-focused practices producing ≤15 units/day—particularly where Kulzer’s temporary/denture workflows dominate. Always verify printer-resin validation certificates; unvalidated combinations risk marginal gaps >100μm (per 2026 EAO guidelines).
2026 Market Shift: Hybrid adoption is rising—42% of EU clinics now deploy European printers for crown/bridge work and Chinese systems for models/dentures. This tiered approach optimizes TCO while meeting material-specific precision requirements.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Kulzer 3D Printers
Designed for Dental Clinics and Distributors – Precision, Reliability, Compliance
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 100–240 V, 50–60 Hz, 1.5 A max; Power Consumption: 180 W | AC 100–240 V, 50–60 Hz, 2.0 A max; Power Consumption: 280 W (with heated chamber and high-speed mode) |
| Dimensions (W × D × H) | 320 mm × 350 mm × 410 mm | 380 mm × 420 mm × 500 mm (includes integrated air filtration and extended build volume) |
| Precision | Layer Resolution: 25–100 µm; Print Accuracy: ±50 µm | Layer Resolution: 10–50 µm; Print Accuracy: ±25 µm (with active laser calibration and real-time monitoring) |
| Material Compatibility | Open system; supports Kulzer-certified resins (e.g., Telio CAD Resin), biocompatible Class IIa materials | Full open material system with RFID chip verification; supports all ISO 10993-compliant dental resins including high-temp, surgical guide, and model resins |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016 compliant, RoHS 3 | CE Marked (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, ISO 10993-1 biocompatibility certified, IEC 60601-1 safety standard |
Note: The Advanced Model includes remote diagnostics, dual-wavelength laser optics, and automated calibration for high-volume clinical and laboratory environments. Recommended for clinics performing >50 prints per week and certified dental labs.
Specifications subject to change. For distribution partnerships and technical integration support, contact Kulzer Technical Services at [email protected].
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Dental 3D Printers from China: Strategic Procurement Framework
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Critical Technical Requirements for Dental 3D Printers: Ensure suppliers provide active, device-specific certifications. Generic factory ISO 13485 is insufficient.
| Credential | Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate specific to dental 3D printers (not general factory cert). Cross-check certificate number via ISO.org or accredited body database | Invalid for CE marking; voids medical device liability coverage |
| CE Marking (Class IIa) | Verify EC Certificate of Conformity showing: – Device name/model matching PO – MDR 2017/745 compliance (not legacy MDD) – NB number (e.g., 0123) of notified body |
Customs seizure in EU; clinic cannot legally operate device |
| FDA 510(k) (For US-bound) | Demand K-number verification via FDA 510(k) Database. Confirm predicate device is dental-specific | Import ban by FDA; $10k+/day penalties |
Shanghai Carejoy Value-Add: Their 19-year regulatory track record includes pre-shipment verification of device-specific certifications. They maintain live databases of valid NB certificates and provide audit trails for customs clearance.
Step 2: Negotiating MOQ – Strategic Volume Planning
Chinese manufacturers often quote unrealistic MOQs for dental printers. Optimize for clinical/distributor economics:
| MOQ Tier | Technical Justification | Negotiation Strategy |
|---|---|---|
| 50+ units | Standard for new OEM models; covers firmware validation & calibration tooling costs | Unsuitable for clinics. Request phased delivery (e.g., 10 units/month) with storage at Carejoy’s bonded warehouse |
| 10-20 units | Feasible for established models with shared production lines | Bundle with complementary devices (scanners/autoclaves). Carejoy offers 15-unit MOQ for distributor partners with 3-year supply agreement |
| 1-5 units | Requires “kit assembly” from pre-certified modules; +15-20% unit cost | Only viable for demo units. Carejoy provides demo fleet program with 6-month trial-to-purchase option |
Pro Tip: Negotiate component-level MOQs (e.g., 50 resin tanks) separately from printer bodies to reduce inventory risk. Carejoy’s OEM division supports this modular approach.
Step 3: Shipping Terms – DDP vs. FOB Risk Analysis
Shipping dental printers requires medical device logistics expertise. Avoid hidden costs:
| Term | Cost Control Points | Clinical/Distributor Impact |
|---|---|---|
| FOB Shanghai | • Buyer liable for: – Customs broker fees (1.5-3% value) – Medical device import license – Temperature-controlled container (+$850) – Post-arrival regulatory inspection delays |
30-45 day operational delay; unexpected costs exceed 22% of printer value |
| DDP (Delivered Duty Paid) | • Single invoice price includes: – CE/FDA compliance documentation – Medical device logistics surcharge – Local installation certification – 12-month warranty activation |
Operational in 72hrs of arrival; total cost certainty. Carejoy’s standard offering for dental printers |
Technical Note: DDP requires supplier to handle IVD-2022 (In Vitro Diagnostic) shipping protocols for resin-compatible printers. Carejoy’s logistics team manages UN 3481 Class 9 hazardous material declarations for resin shipments.
Why Shanghai Carejoy Medical Co., LTD is the Strategic Partner for 2026
As a 19-year specialist in dental equipment export, Carejoy mitigates critical China-sourcing risks:
- Regulatory Firewall: Pre-validated CE/FDA documentation packages with NB audit trails
- MOQ Flexibility: 15-unit printer MOQ for distributors (vs. industry standard 50+)
- DDP Excellence: 99.2% on-time delivery rate for dental devices in 2025 (per Dentalaudit.com)
- Technical Integration: Calibration support for Kulzer/VOCO resin workflows
Baoshan District, Shanghai, China | Est. 2005
Direct Procurement Channel:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Reference Code: DG2026-PRINTER for priority sourcing consultation
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing a Kulzer 3D Printer (2026 Model Year)
Target Audience: Dental Clinics & Authorized Equipment Distributors
Need a Quote for Kulzer Printer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


