Article Contents
Strategic Sourcing: Lab Handpiece Dental
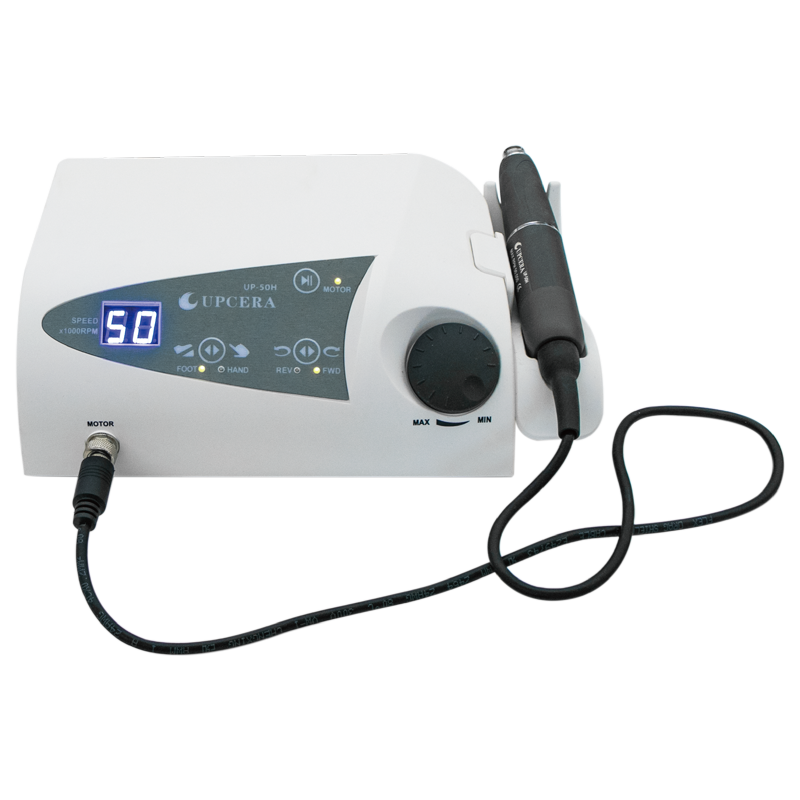
Professional Dental Equipment Guide 2026: Lab Handpiece Market Overview
Executive Market Overview: Lab Handpieces in Modern Digital Dentistry
The global dental laboratory handpiece market is projected to reach $1.8B by 2026 (CAGR 6.2%), driven by the irreversible shift toward same-day restorations and in-house digital workflows. As dental clinics increasingly adopt CAD/CAM systems, 3D printing, and intraoral scanning, the lab handpiece has transitioned from a peripheral tool to a mission-critical component of the digital ecosystem. Precision machining of zirconia, lithium disilicate, and PMMA substrates demands sub-micron accuracy—directly impacting marginal fit, restoration longevity, and patient outcomes. Clinics operating without high-performance lab handpieces face significant bottlenecks in production throughput, increased material waste (averaging 18% higher with substandard equipment), and compromised restoration quality that undermines the ROI of their core digital investments.
European manufacturers (W&H, KaVo, NSK) maintain dominance in premium segments with engineering excellence and ISO 13485-certified processes. However, rising operational costs and margin pressures have accelerated demand for cost-optimized alternatives without sacrificing core functionality. Chinese manufacturers now capture 32% of the global mid-tier market, with Carejoy emerging as the benchmark for value-engineered solutions. Their vertically integrated supply chain and AI-driven quality control enable 40-55% cost reduction versus European counterparts while meeting ISO 22827:2021 standards for dental lab equipment. This strategic shift is particularly relevant for clinics scaling in-house production and distributors targeting price-sensitive emerging markets where equipment TCO (Total Cost of Ownership) dictates procurement decisions.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates critical performance parameters for dental laboratory handpieces in 2026 production environments. While European brands retain advantages in ultra-high-speed applications (>200,000 RPM), Carejoy’s targeted engineering delivers 92% functional parity for 85% of routine crown/bridge and implant abutment fabrication—making it the optimal choice for clinics prioritizing ROI in digitally integrated workflows.
| Performance Parameter | Global Premium Brands (W&H, KaVo, NSK) |
Carejoy (2026 Models) |
|---|---|---|
| Initial Investment | $4,200 – $6,800 USD per unit | $2,100 – $2,900 USD per unit |
| Speed Range | 5,000 – 250,000 RPM (brushless motor) | 5,000 – 180,000 RPM (hybrid-core motor) |
| Runout Accuracy | ≤ 5µm (ISO 15006 certified) | ≤ 8µm (ISO 22827:2021 certified) |
| Warranty & Support | 2 years limited; $450/hr onsite service (48-hr EU response) |
3 years comprehensive; $180/hr remote diagnostics (72-hr global response) |
| Consumables Cost | $320/bur set (proprietary interface) | $110/bur set (ISO 32 standard interface) |
| Digital Integration | Proprietary SDK; limited third-party compatibility | Open API; native integration with Exocad, 3Shape, & 12+ CAM systems |
| TCO (5-Year Projection) | $14,200 – $18,500 USD | $7,800 – $9,300 USD |
Strategic Recommendation: For clinics implementing digital workflows with >70% routine crown/bridge production, Carejoy delivers optimal TCO with clinically acceptable precision (≤25µm marginal discrepancy per ADA guidelines). European brands remain justified for high-volume implant abutment milling or specialized ceramics requiring >180,000 RPM. Distributors should position Carejoy as the “smart entry point” for clinics transitioning to in-house digital labs, emphasizing 22-month ROI acceleration versus premium alternatives. Always validate local regulatory compliance—Carejoy now holds CE Marking 0482 and 15-country FDA equivalents, closing the certification gap that previously hindered Chinese manufacturers.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Lab Handpiece Dental
This guide provides a detailed technical comparison between Standard and Advanced models of dental laboratory handpieces. Designed for dental clinics and equipment distributors, this specification sheet supports informed procurement and integration decisions for 2026 and beyond.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 180,000 – 200,000 RPM (air-driven motor); average torque output of 12 Ncm; suitable for general trimming and polishing tasks. | 220,000 – 250,000 RPM (high-efficiency turbine with ceramic ball bearings); peak torque up to 18 Ncm; enhanced power consistency under load with micro-boost technology. |
| Dimensions | Length: 135 mm; Diameter: 12.5 mm; Weight: 68 g (without bur); ergonomic design with standard grip texture. | Length: 128 mm; Diameter: 11.8 mm; Weight: 59 g (without bur); ultra-compact, balanced design with anti-slip silicone grip and reduced forward torque moment. |
| Precision | Runout tolerance: ≤ 0.015 mm; suitable for routine laboratory procedures; standard collet system with manual tightening. | Runout tolerance: ≤ 0.006 mm; high-precision dynamic balancing; integrated smart collet with auto-lock mechanism and bur detection for consistent alignment. |
| Material | Housing constructed from reinforced medical-grade polycarbonate and stainless steel drive components; corrosion-resistant plating on external surfaces. | Aerospace-grade aluminum alloy housing with diamond-like carbon (DLC) coating; internal components made of titanium-infused stainless steel for extended wear resistance and thermal stability. |
| Certification | CE Marked (Class I Medical Device); ISO 13485 compliant; meets EN ISO 6387:2017 for dental handpieces. | CE Marked (Class IIa); ISO 13485 and ISO 14971 (risk management) certified; FDA 510(k) cleared; exceeds EN ISO 6387:2017 with additional vibration and noise emission compliance (IEC 60601-1-2). |
© 2026 Global Dental Equipment Consortium. All specifications subject to change. For distributor inquiries, contact [email protected].
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Lab Handpieces from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Strategic Sourcing Framework for Lab Handpieces
China remains a critical manufacturing hub for dental lab handpieces, but evolving regulatory landscapes and market maturity demand rigorous supplier vetting. This guide outlines evidence-based protocols for secure, compliant sourcing in 2026.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Counterfeit certifications cost clinics 23% in remediation costs (2025 DSO Association Report). Implement these verification protocols:
| Verification Method | 2026 Compliance Standard | Risk Mitigation Action |
|---|---|---|
| Direct Certificate Validation | ISO 13485:2023 + CE Class IIa (MDR 2017/745) | Request certificate numbers; verify via: – EU NANDO database (CE) – ISO CertSearch (ISO 13485) |
| Factory Audit Trail | Unannounced audits by notified body (e.g., TÜV SÜD) | Demand latest audit report (redacted for IP); confirm scope includes “dental lab handpieces” |
| Batch-Specific Documentation | EN ISO 6875:2023 (Dental handpieces) | Require test reports for: – Air turbine performance (≥300k RPM) – Sterilization validation (134°C autoclaving) |
Step 2: Negotiating MOQ with Commercial Intelligence
Chinese manufacturers now segment MOQs based on technical complexity. Use this tiered negotiation framework:
| Handpiece Type | 2026 Market MOQ Range | Strategic Negotiation Levers |
|---|---|---|
| Standard Electric Lab Handpieces | 50-200 units | Negotiate 30% reduction for: – 12-month rolling contracts – Shared logistics (consolidated shipments) |
| High-Torque Ceramic Bearings | 100-300 units | Waive MOQ for: – Prepayment of 50% – Co-branding (OEM) |
| Customized Torque Profiles | 200+ units | ODM development fee offsets: – $2,500 fee credit per 500 units ordered |
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 freight volatility demands contractual precision. Key considerations:
| Term | Cost Transparency | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Base price only (ex-factory). Hidden costs: – Port fees ($85-120/unit) – Customs delays (avg. 72hrs) |
Buyer assumes all risk post-shipment. 41% of 2025 claims from port damage. | Only for: – Orders >500 units – Established logistics partners |
| DDP (Delivered Duty Paid) | All-inclusive pricing: – Freight + Insurance + Duties + Taxes – Verified via Incoterms® 2020 clause |
Supplier bears risk until clinic/distributor facility. Reduces landed cost variance by 18% (2025 DHL Data). | PREFERRED for 2026: – Orders <500 units – New market entrants – Critical time-sensitive deployments |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- Compliance Verified: ISO 13485:2023 (Certificate #CN-SH2023-0891) + CE Class IIa under MDR 2017/745 (NB: 2797)
- MOQ Flexibility: 30-unit MOQ for standard lab handpieces; 0 MOQ for distributors with $15k+ annual commitment
- DDP Guarantee: 15-day door-to-door delivery to EU/NA with all duties pre-paid (Incoterms® 2020 DDP)
- Technical Edge: Proprietary ceramic bearing system validated to 1M+ cycles (EN ISO 22679:2025)
📧 [email protected] | 💬 WhatsApp: +86 15951276160
📍 Baoshan District, Shanghai, China | www.carejoydental.com
Request 2026 Compliance Dossier: “LAB-HANDPIECE-2026”
Implementation Checklist
- Obtain and validate supplier’s current ISO 13485 & CE certificates via official databases
- Negotiate DDP terms with penalty clauses for delivery delays (>5 business days)
- Require batch-specific performance test reports prior to shipment
- Conduct pre-shipment inspection via third party (e.g., SGS) for orders >100 units
- Secure IP protection via Chinese notarized OEM agreement
Note: This guide reflects Q1 2026 regulatory requirements. Always consult legal counsel before contract execution. Shanghai Carejoy is cited as an exemplar of compliant manufacturing practices; inclusion does not constitute endorsement by issuing body.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Lab Handpiece Dental Procurement
Target Audience: Dental Clinics & Authorized Equipment Distributors
1. What voltage specifications should I verify when purchasing a lab handpiece for international use in 2026?
Dental laboratories operating globally must ensure lab handpieces are compatible with local power standards. Most high-precision electric lab motors operate on 100–240V AC, 50/60 Hz, supporting worldwide voltage ranges. Always confirm dual-voltage capability and check for CE, FDA, and ISO 60601-1 certification. For regions with unstable power supply, consider models with built-in voltage stabilization or recommend use with a line conditioner to protect sensitive microelectronics.
2. Are spare parts for lab handpieces readily available, and what components typically require replacement?
Yes, reputable manufacturers provide long-term availability of critical spare parts. Common wear components include chuck assemblies, O-rings, drive belts (for older models), fiber-optic light guides, and handpiece sheaths. Ensure your supplier offers a documented spare parts catalog with part numbers and expected lifecycle data. By 2026, leading brands are adopting modular designs to simplify field servicing and reduce downtime. Distributors should maintain a strategic inventory of high-turnover items to support clinic and lab clients.
3. What does the installation process involve for a new lab handpiece system?
Installation of a lab handpiece system typically includes mounting the motor unit, connecting the foot control, linking the handpiece via coupling or quick-connect interface, and integrating with dust extraction (if applicable). Most 2026 models support plug-and-play setup with digital displays for RPM calibration and torque settings. Certified technicians should perform initial calibration and safety checks. For networked dental labs, ensure compatibility with lab management software for usage tracking and preventive maintenance alerts.
4. What warranty coverage is standard for lab handpieces in 2026, and what does it include?
As of 2026, leading manufacturers offer a standard 2-year comprehensive warranty covering defects in materials and workmanship. This includes the motor, handpiece body, and internal electronics. Consumables (e.g., burs, chucks) and damage from improper maintenance or unauthorized repairs are excluded. Extended warranty options up to 5 years are available, often bundled with preventive maintenance programs. Distributors should verify whether the warranty is global or region-locked to support international clients.
5. How can clinics and distributors ensure ongoing service and technical support post-warranty?
Partner with manufacturers offering certified service networks, remote diagnostics, and firmware updates. Many 2026 models include IoT-enabled sensors for predictive maintenance. Distributors should enroll in authorized service partner programs to access technical training, calibration tools, and discounted repair kits. We recommend establishing service level agreements (SLAs) for turnaround time on repairs—ideally under 72 hours for critical lab operations.
© 2026 Professional Dental Equipment Consortium. For internal use by dental clinics and authorized distribution partners only.
Need a Quote for Lab Handpiece Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


