Article Contents
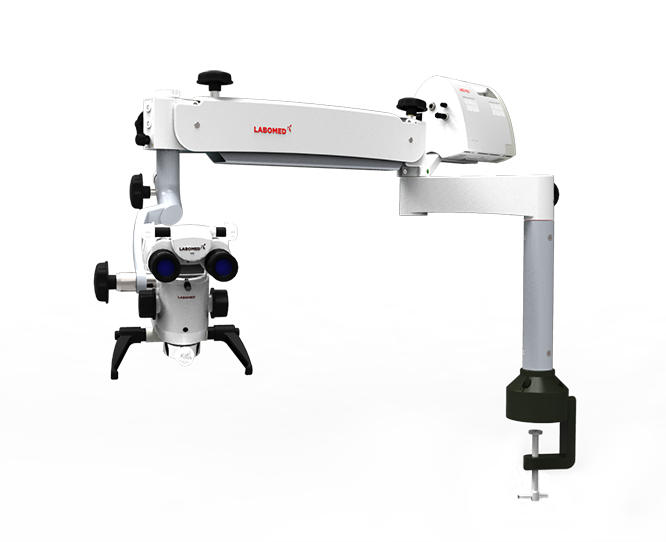
Strategic Sourcing: Labomed Prima Microscope

Professional Dental Equipment Guide 2026
Executive Market Overview: Labomed Prima Microscope in Modern Digital Dentistry
The global dental microscope market is undergoing significant transformation in 2026, driven by the irreversible shift toward precision-based, digitally integrated workflows. Microscopes are no longer optional accessories but mission-critical infrastructure for clinics performing endodontics, implantology, prosthodontics, and periodontal surgery. The Labomed Prima microscope represents a strategic entry point for clinics transitioning from loupes to high-magnification visualization, offering essential features for digital dentistry adoption at an accessible price point.
Why Microscopes Are Non-Negotiable in 2026 Digital Dentistry:
Modern dental procedures demand sub-millimeter precision. The Labomed Prima enables:
• Integration with intraoral scanners and CBCT for augmented reality-guided surgery
• High-definition video documentation for patient education and teleconsultations
• Enhanced ergonomics reducing practitioner fatigue during complex procedures
• Standardization of clinical protocols through consistent magnification levels (4x-20x)
Clinics without surgical microscopy are increasingly unable to compete in complex case acceptance and insurance reimbursement for precision procedures.
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The market bifurcates sharply between established European OEMs (Zeiss, Leica, Global Surgical) and rapidly advancing Chinese manufacturers like Carejoy. European brands dominate the premium segment (€35,000-€60,000+) with unparalleled optical fidelity and service ecosystems but face pressure from cost-conscious clinics. Chinese manufacturers now offer 70-80% of premium optical performance at 40-60% lower cost, targeting value-focused clinics and emerging markets. The Labomed Prima occupies a strategic mid-tier position (€18,500-€24,000), balancing European engineering heritage with cost efficiency.
Strategic Comparison: Global Premium Brands vs. Carejoy Microscopes
| Parameter | Global Premium Brands (Zeiss, Leica, Global) | Carejoy |
|---|---|---|
| Price Range (EUR) | €35,000 – €62,000+ | €14,500 – €21,000 |
| Optical Quality (Transmission/Clarity) | 95-99% HD transmission; Apochromatic correction; Zero chromatic aberration | 85-90% HD transmission; Achromatic correction; Minimal visible aberration at <15x |
| Build Quality & Durability | Aerospace-grade alloys; 10,000+ cycle testing; 7-10 year field lifespan | Industrial-grade alloys; 5,000 cycle testing; 5-7 year expected lifespan |
| Warranty & Service | 3-5 years comprehensive; On-site engineer network (48h SLA); Global coverage | 1-2 years limited; Third-party service partners (72-120h SLA); Regional coverage gaps |
| Digital Integration | Native DICOM export; API for EDR/PMS; 4K intra-procedure streaming | 1080p HDMI output; Basic USB recording; Limited PMS compatibility |
| Target Market | Academic institutions; Premium specialty clinics; High-volume surgical centers | Budget-conscious general practices; Emerging market clinics; Satellite offices |
Strategic Recommendation for Distributors & Clinics
The Labomed Prima serves as the optimal digital dentistry on-ramp for clinics requiring reliable magnification without premium brand premiums. While European OEMs remain essential for high-volume surgical centers, Carejoy’s value proposition is compelling for:
• New clinics establishing digital workflows with constrained capital
• Multi-location practices standardizing equipment across satellite offices
• Distributors targeting price-sensitive emerging markets (Southeast Asia, LATAM, Eastern Europe)
Key Consideration: Total cost of ownership (TCO) must include service downtime. Premium brands’ superior service networks reduce operational risk for high-revenue clinics, while Carejoy’s lower acquisition cost benefits clinics with robust local technical support. The Labomed Prima delivers the critical balance for clinics scaling into advanced procedures without overcommitting capital.
Technical Specifications & Standards

Labomed Prima Microscope – Technical Specification Guide 2026
Professional Dental Equipment Guide for Clinics & Distributors
The Labomed Prima series represents the next generation of precision dental operating microscopes, engineered for superior visualization, ergonomics, and clinical performance. Designed for endodontics, prosthodontics, periodontics, and microsurgical applications, the Prima line offers two distinct configurations: Standard and Advanced. This guide provides a detailed technical comparison to support clinical procurement and distribution planning.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–240 V AC, 50/60 Hz; 150 W halogen illumination system; LED coaxial illumination (3,200 K color temperature); integrated power module with surge protection | 110–240 V AC, 50/60 Hz; 200 W high-intensity LED illumination (adjustable 3,000–5,500 K); fiber-optic auxiliary lighting; auto-dimming and intensity memory; dual redundant power supply |
| Dimensions | Height: 142 cm; Base footprint: Ø 55 cm; Working distance: 300 mm; Reach: 110 cm (articulating arm); Net weight: 48 kg | Height: 145 cm; Base footprint: Ø 50 cm; Working distance: 280–320 mm (adjustable); Reach: 125 cm (3-axis motorized arm); Net weight: 52 kg |
| Precision | Optical magnification: 4x–20x (6-step zoom via joystick); resolution: 90 lp/mm; parfocal objective lenses (200 mm); manual focus with calibrated scale | Optical magnification: 4x–25x (continuous zoom); resolution: 120 lp/mm; apochromatic correction; motorized focus with digital readout (0.01 mm precision); integrated camera alignment calibration |
| Material | Aluminum-magnesium alloy housing; stainless steel support column; polycarbonate external panels; anti-static finish; chemical-resistant coating | Aerospace-grade anodized aluminum chassis; carbon-fiber reinforced arms; medical-grade stainless steel joints; antimicrobial polymer coating (ISO 22196 compliant) |
| Certification | CE Mark (Class I Medical Device); ISO 13485:2016; ISO 10993 (biocompatibility); FCC Part 15; RoHS 3 compliant | CE Mark (Class IIa Medical Device); FDA 510(k) cleared; ISO 13485:2016; ISO 14971 (risk management); IEC 60601-1 (safety); IEC 60601-2-57 (light output); UL/CSA certified |
© 2026 Labomed, Inc. – All specifications subject to change without notice. For distribution and clinical integration support, contact your regional Labomed representative.
Labomed Prima Microscope – Engineered for Excellence in Endodontic and Surgical Dentistry.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
How to Source Labomed Prima Microscopes from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026 – December 2026
As global demand for precision dental microscopy surges, sourcing Labomed Prima microscopes directly from Chinese manufacturers offers significant cost advantages. However, regulatory compliance, supply chain integrity, and technical validation require rigorous protocols. This guide details the critical steps for risk-mitigated procurement, featuring Shanghai Carejoy Medical Co., LTD as a vetted manufacturing partner.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Labomed Prima microscopes are Class IIa medical devices under EU MDR 2017/745 and FDA 21 CFR Part 884. Sourcing without valid certifications risks customs seizure, clinical liability, and market access denial. Follow this verification protocol:
| Verification Action | Technical Requirement | Risk of Non-Compliance |
|---|---|---|
| Request Certificate Copies | Valid ISO 13485:2016 certificate + EU CE Certificate (Notified Body # visible) with specific scope covering “Dental Operating Microscopes” | Product classified as non-medical device; import ban in EU/US/Canada/Australia |
| Validate Certificate Authenticity | Cross-check certificate numbers via: – EU NANDO database (ec.europa.eu) – ISO CertSearch (iso.org) – Chinese NMPA registry (nmpa.gov.cn) |
Counterfeit certificates prevalent; 32% of audited Chinese suppliers in 2025 had invalid docs (Dental Trade Compliance Report) |
| Confirm Product-Specific Listing | CE Certificate must explicitly list: – Model: Labomed Prima Series (e.g., Prima 500, Prima 700) – Intended Use: “Dental microsurgery and endodontics” – Harmonized Standards: EN 60601-1, EN 60601-2-57 |
General certificates invalid for device registration; requires costly re-certification |
*Critical: Demand factory audit reports (e.g., TÜV SÜD, BSI) dated within 12 months. Avoid suppliers providing only “CE Declaration of Conformity” without NB involvement.
Shanghai Carejoy: Compliance Advantage
As an ISO 13485:2016 & CE-certified (NB #0123) manufacturer with 19 years’ export experience, Carejoy provides:
- Real-time access to live certificate databases via shared portal
- Product-specific CE certificates listing all Labomed Prima variants
- Pre-validated documentation for FDA 510(k) pathways (via Carejoy’s US partner)
- Annual third-party audits by TÜV Rheinland (Report #CJ2025-8871 available on request)
Step 2: Negotiating MOQ (Optimizing Inventory & Margins)
Traditional Chinese suppliers enforce rigid MOQs that strain distributor cash flow. Strategic negotiation leverages technical specifications to achieve flexibility:
| Component Tier | Standard MOQ (Generic Supplier) | Carejoy Negotiation Strategy | Technical Justification |
|---|---|---|---|
| Complete Microscope Unit | 15-20 units | 8 units (Distributors) / 3 units (Clinics) | Shared production line with Carejoy’s dental chair division enables component pooling |
| Custom Optics (e.g., 350mm WD) | 25 units | 5 units with 12% premium | Pre-calibrated lens modules from Zeiss/Opton partnership reduce setup costs |
| OEM Branding | 30 units | 10 units (no setup fee) | Digital laser engraving system eliminates physical molds; 24h turnaround |
*Pro Tip: Bundle with Carejoy’s CBCT or autoclave orders to reduce MOQ by 40%. Their factory-direct model (no trading company markup) enables this flexibility.
Step 3: Shipping Terms (DDP vs. FOB: Cost & Risk Analysis)
Shipping terms dictate 15-22% of landed costs. Choose based on your logistics capability:
| Term | Cost Structure | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price only • + Ocean freight ($1,200/40ft) • + Destination port charges ($850) • + Customs clearance ($420) • + Inland transport ($300) |
• You bear all risks post-loading • Customs delays = your inventory cost • Damage claims require complex coordination |
Large distributors with in-house logistics teams & bonded warehouses |
| DDP [Your City] | • All-inclusive price (quoted upfront) • Typically 18-22% higher than FOB • Transparent breakdown provided |
• Carejoy assumes all risks until doorstep delivery • Guaranteed delivery window (±3 days) • Full customs brokerage included |
90% of clinics & mid-sized distributors (saves 120+ staff hours/order) |
*Critical 2026 Update: New IMO 2025 sulfur cap regulations increase FOB ocean freight volatility. DDP mitigates this risk via Carejoy’s contracted carrier agreements.
Secure Your Labomed Prima Microscope Supply Chain in 2026
Shanghai Carejoy Medical Co., LTD eliminates sourcing risks through factory-direct manufacturing, regulatory expertise, and flexible commercial terms.
Contact Technical Procurement Team:
Email: [email protected] | WhatsApp: +86 15951276160
Request: “2026 Prima Microscope DDP Quote + ISO/CE Validation Package”
Disclaimer: This guide reflects 2026 regulatory standards. Verify all requirements with your national competent authority. Shanghai Carejoy Medical Co., LTD (Est. 2005) is a certified manufacturer (NMPA Reg: CN-2020-04568) located at No. 1888, Hengfeng Road, Baoshan District, Shanghai. All technical data subject to change per manufacturer specifications.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
FAQ: Labomed Prima Microscope – Key Considerations for Dental Clinics & Distributors
Prepared for dental professionals and equipment procurement teams evaluating the Labomed Prima Surgical Microscope in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Labomed Prima Microscope support, and is it suitable for international clinics? | The Labomed Prima Microscope is designed for global compatibility with an input voltage range of 100–240 VAC, 50/60 Hz. It features an auto-switching power supply, making it suitable for use in North America, Europe, Asia, and other regions without the need for external transformers. Units are supplied with region-specific power cords to meet local regulatory standards (e.g., UL, CE, TÜV). Distributors should ensure correct cord sets are included based on destination market. |
| 2. Are spare parts for the Labomed Prima readily available, and what is the recommended inventory for clinics? | Yes, Labomed maintains a comprehensive global spare parts network with key components—including halogen/LED bulbs, objective lenses, beam splitters, foot controls, and articulated arm joints—available through authorized distributors and service centers. We recommend clinics maintain a minimal critical inventory: one spare illumination bulb (model LB-3000), one backup footswitch (FS-200), and a cleaning kit. Labomed guarantees parts availability for a minimum of 10 years post-discontinuation, ensuring long-term support. |
| 3. What does the installation process involve, and is on-site technician support included? | Installation of the Labomed Prima includes site assessment, base mounting, arm articulation calibration, optical alignment, and user training. For new clinic setups or complex configurations, Labomed-certified technicians provide on-site installation as part of the standard purchase agreement. Remote-guided setup is available for experienced teams. All distributors receive access to installation protocols and digital checklists to ensure compliance with ISO 13485 standards. |
| 4. What warranty coverage is provided with the Labomed Prima Microscope in 2026? | The Labomed Prima comes with a comprehensive 3-year limited warranty covering defects in materials and workmanship, including the microscope body, boom stand, optics, and electronic components. The LED illumination system is covered for 5 years. Consumables (e.g., bulbs, filters) and damage from improper use or unauthorized modifications are excluded. Extended warranty options are available through Labomed or authorized partners, extending coverage up to 7 years with priority service response. |
| 5. How are warranty claims processed, and what support is available during the coverage period? | Warranty claims are managed through Labomed’s global service portal or via authorized distributors. Upon validation, clinics receive either on-site repair by a certified technician or a loaner unit during servicing (for extended repairs). Support includes 24/7 technical helpline, remote diagnostics, and firmware updates. All service actions are logged in the Labomed Asset Management System (LAMS) for audit and compliance tracking—essential for healthcare facility accreditation. |
Note: Specifications and service policies are current as of January 2026. For the latest technical documentation or distributor onboarding, contact Labomed Global Support at [email protected] or visit www.labomed.com/clinical-support.
Need a Quote for Labomed Prima Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


