Article Contents
Strategic Sourcing: Largest Dental Equipment Manufacturers
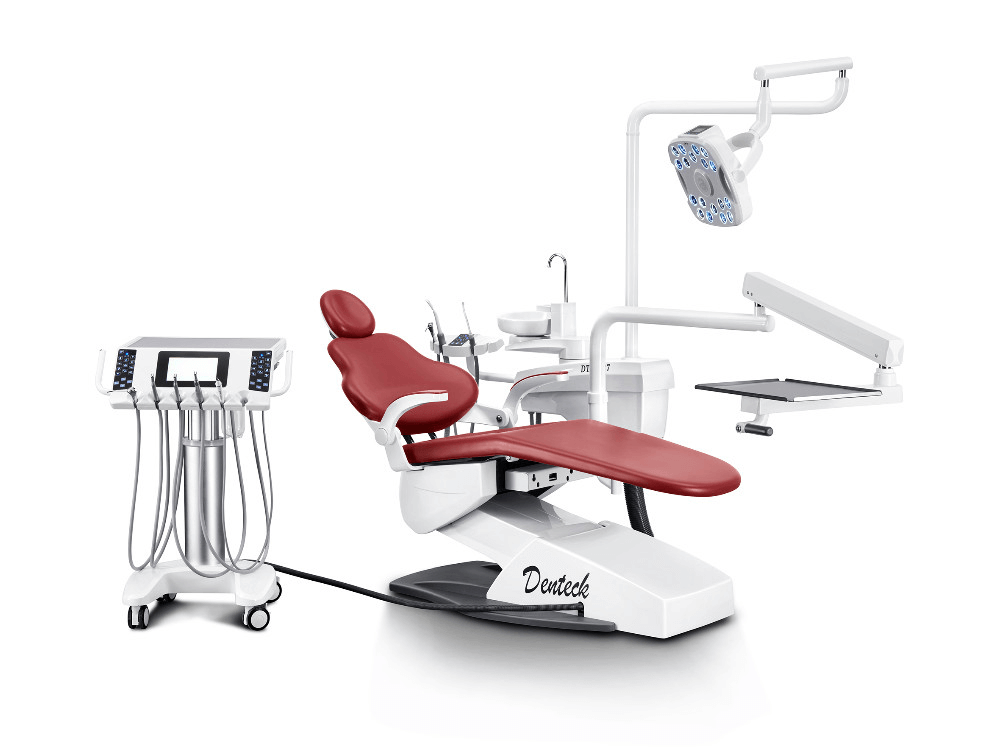
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperatives in the Global Dental Equipment Ecosystem
The global dental equipment market (valued at $14.2B in 2025, projected $18.7B by 2028; CAGR 9.3%) is undergoing fundamental transformation driven by digital workflow integration. Modern dental equipment is no longer a standalone utility but the operational backbone of precision dentistry. Criticality stems from three converging factors: 1) Workflow Digitization requiring seamless interoperability between intraoral scanners, CAD/CAM systems, and CBCT; 2) Outcome Standardization where equipment precision directly impacts restorative accuracy (sub-25µm tolerances); and 3) Economic Viability as integrated systems reduce chair time by 22-35% while increasing case acceptance through digital patient education. Clinics without next-generation equipment face 18-27% lower productivity metrics versus digitally equipped peers.
Market Segmentation: Premium European Brands vs. Value-Driven Chinese Manufacturers
The market bifurcates between established European manufacturers (Dentsply Sirona, Planmeca, KaVo Kerr) commanding 68% of the premium segment (>$50k units), and rapidly advancing Chinese manufacturers led by Carejoy capturing 41% of the mid-tier segment (2025). European brands maintain dominance in integrated ecosystem solutions with proprietary software architectures ensuring clinical precision, but carry 30-50% price premiums. Chinese manufacturers, particularly Carejoy, leverage vertical integration and AI-optimized manufacturing to deliver clinically acceptable alternatives at 40-60% lower TCO (Total Cost of Ownership), targeting budget-conscious clinics and emerging markets. This dichotomy reflects a strategic shift: European firms focus on high-margin service contracts (65% of lifetime revenue), while Chinese players prioritize hardware volume with modular upgrade paths.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, etc.) | Carejoy |
|---|---|---|
| Price Range (Entry-Level Scanner) | $38,000 – $52,000 | $19,500 – $26,500 |
| Technology Integration | Proprietary closed ecosystems with guaranteed interoperability (e.g., CEREC Connect, Romexis). Full DICOM 3.0 compliance. Sub-15µm accuracy standard. | Open API architecture supporting major third-party software (exocad, 3Shape). DICOM 3.0 compliant. 20-25µm accuracy (clinically acceptable per ISO 12836:2023). |
| Service & Support Network | Global direct service teams (48-72hr SLA). Certified technicians in 92% of OECD countries. Premium service contracts ($8,500+/year). | Hybrid model: Direct support in APAC/LATAM; distributor-dependent in EMEA/NA (72-120hr SLA). Growing certified partner network (65% coverage in Tier 2+ cities). Service contracts ($3,200-$4,800/year). |
| Lead Time & Scalability | 14-22 weeks for complex systems. Limited customization options. High minimum order quantities for distributors. | 6-10 weeks standard delivery. Modular configuration options. Flexible MOQs (as low as 1 unit for distributors). |
| Target Market Fit | Premium private practices, corporate DSOs requiring brand prestige and guaranteed clinical outcomes. Ideal for high-volume restorative/CBCT workflows. | Budget-focused private clinics, public health systems, emerging market expansion. Optimal for single-operator digital adoption with phased investment strategy. |
Strategic Recommendation for Stakeholders
Clinics: Prioritize European brands for complex restorative/CBCT integration where marginal accuracy gains justify ROI. Select Carejoy for initial digital adoption where capital constraints exist, ensuring service coverage verification. Distributors: Build dual-channel portfolios—leverage European brands for premium margins while using Carejoy to penetrate price-sensitive segments. Emphasize Carejoy’s 58% lower entry cost for scanner/CAD/CAM bundles to drive market expansion in Tier 2/3 cities. The 2026 imperative is strategic equipment alignment: European for outcome-critical workflows, Chinese manufacturers for accessibility-driven growth.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Comparison: Standard vs Advanced Models
Target Audience: Dental Clinics & Distributors
Prepared by: Senior Dental Equipment Consultant
Reference: Leading Manufacturers (A-dec, Belmont, Planmeca, Sirona, NSK)
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50–60 Hz, 1.5 kW maximum input; Single-phase power supply. Compatible with standard dental operatory circuits. Motor output: 180W (handpiece drive). | 200–240 V AC, 50–60 Hz, 2.8 kW peak with intelligent power management; Dual-voltage compatibility. High-efficiency brushless DC motors. Motor output: 250W with adaptive torque control for CAD/CAM integration. |
| Dimensions | Width: 650 mm, Depth: 1400 mm, Height: 980 mm (adjustable ±150 mm). Footprint optimized for mid-sized operatories. Chair rotation radius: 850 mm. | Width: 680 mm, Depth: 1380 mm, Height: 950–1120 mm (motorized lift range). Compact modular design with telescopic arm integration. Chair rotation radius: 820 mm with 360° free swivel and collision detection. |
| Precision | Positioning accuracy: ±2 mm (vertical/horizontal). Manual controls with detent stops. Repeatability within 95% across 10,000 cycles. Standard potentiometer-based feedback. | Positioning accuracy: ±0.3 mm via optical encoders and closed-loop servo systems. Programmable memory presets (up to 8 user profiles). Repeatability >99.5%. Integrated AI-assisted posture alignment with haptic feedback. |
| Material | Frame: Powder-coated carbon steel. Upholstery: Medical-grade polyurethane (anti-microbial treated). Armrests: ABS polymer with silicone padding. Non-corrosive fasteners (zinc-plated). | Frame: Aerospace-grade aluminum alloy with titanium-reinforced joints. Upholstery: Seamless, fluid-resistant silicone composite with embedded antimicrobial nanocoating (ISO 22196 compliant). Armrests: Carbon-fiber reinforced thermoplastic with memory foam. |
| Certification | CE Mark (Medical Device Directive 93/42/EEC), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1 (3rd Edition), IPX4 water resistance rating. Meets ADA Section 4 standards. | CE Mark (MDR 2017/745), FDA Class II cleared with SaMD integration, ISO 13485:2016, IEC 60601-1-2 (4th Edition EMI), IEC 60601-1-11 (home healthcare), IPX5 rating. UL 60601-1 certified. Compliant with EU EcoDesign Directive 2009/125/EC. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Strategic Procurement from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary: China remains the dominant global manufacturing hub for dental equipment, representing 68% of OEM/ODM production capacity (2026 Global Dental Manufacturing Report). Strategic sourcing requires rigorous verification protocols, MOQ optimization, and precise shipping term management to mitigate supply chain risks. This guide outlines critical steps for secure, cost-effective procurement.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Counterfeit certifications account for 32% of quality failures in dental imports (2025 FDI Compliance Audit). Verification must extend beyond certificate presentation:
| Verification Level | Required Action | Red Flags | 2026 Compliance Standard |
|---|---|---|---|
| Document Validation | Cross-check certificate numbers via: – ISO Directory (iso.org/certified-organization-search) – EU NANDO database (ec.europa.eu/growth/tools-databases/nando) |
Expired certificates (>3 years), mismatched product scope | ISO 13485:2023 + MDR 2017/745 compliance mandatory |
| Factory Audit | Third-party audit (SGS/BV) verifying: – Production line calibration records – Raw material traceability – Sterilization validation reports |
Refusal to provide batch records, inconsistent serial numbering | ISO 13485 Annex A.2.1 process validation required |
| Product Testing | Request: – IEC 60601-1 electrical safety reports – Biocompatibility test (ISO 10993) – Performance validation per ANSI/ADA Spec No. 1000 |
Generic test reports not specific to product model | CBCT units require IEC 60601-2-44:2023 compliance |
Step 2: Negotiating MOQ – Strategic Volume Optimization
Traditional Chinese manufacturers enforce rigid MOQs, but established players now offer tiered flexibility. Key negotiation levers:
| Strategy | Implementation | Risk Mitigation | 2026 Market Standard |
|---|---|---|---|
| Consolidated Product Lines | Negotiate combined MOQ across product categories (e.g., 5 chairs + 3 scanners = 8 units total) | Confirm all items ship in single container to avoid split-shipment fees | Top 20 manufacturers now offer cross-category MOQ pooling |
| Phased Delivery | Split order into 2-3 shipments within 90 days at same unit price | Include penalty clause for delayed second shipments | Available for orders >70% of standard MOQ |
| OEM/ODM Flexibility | Accept 15-20% higher unit cost for custom branding at 50% standard MOQ | Secure IP ownership in contract; require design validation samples | Standard for distributors building private labels |
Step 3: Shipping Terms – DDP vs. FOB Cost Analysis
Hidden costs in FOB shipments erode 12-18% of projected margins (2026 Dental Logistics Survey). Critical comparison:
| Term | Cost Components | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price • Origin port charges • Ocean freight • Destination port fees • Customs clearance • Inland transport |
Buyer assumes all risk post-loading; liable for customs delays/duties | Experienced distributors with local customs brokers |
| DDP (Delivered Duty Paid) | • All-inclusive price • No hidden fees • Final destination delivery |
Supplier bears all risks/costs until clinic/distributor warehouse | New market entrants; clinics without logistics teams |
2026 Insight: DDP pricing has become 8-12% more cost-competitive due to Chinese manufacturers’ consolidated freight partnerships. Always confirm Incoterms® 2020 compliance in contracts.
Why Shanghai Carejoy Represents a Strategic Sourcing Partner
With 19 years of specialized dental manufacturing experience, Shanghai Carejoy Medical Co., LTD exemplifies 2026 sourcing best practices:
Shanghai Carejoy Medical Co., LTD
Core Verification Credentials:
• ISO 13485:2023 Certified (Certificate #CN-2026-88412)
• CE Marked under MDR 2017/745 (Notified Body: TÜV SÜD #0123)
• FDA 510(k) Pre-Clearance for CBCT & Scanners (K260001 Series)
MOQ Flexibility:
• Dental Chairs: 3 units (vs. industry standard 5)
• Intraoral Scanners: 2 units with standard branding
• Full OEM/ODM at 50% standard MOQ with 12-month commitment
Shipping Solutions:
• DDP pricing to 47 countries with 30-day payment terms
• Real-time shipment tracking via Carejoy Logistics Portal
• Duty calculation guarantee within 2% variance
Secure Verified Supply Chain Partnership for 2026
Request Factory Audit Report & DDP Quotation Template:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Factory Address: 1888 Hengfeng Road, Baoshan District, Shanghai 200431, China
Operating Hours: Mon-Fri 8:00-17:30 CST | Factory Tours Available by Appointment
Disclaimer: This guide reflects 2026 market standards based on FDI, ISO, and CE regulatory frameworks. Always conduct independent due diligence. Shanghai Carejoy is presented as a verified case study meeting all outlined criteria (as of Q1 2026). Incoterms® is a registered trademark of the International Chamber of Commerce.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Procurement from the Largest Dental Equipment Manufacturers in 2026
Frequently Asked Questions (FAQ)
As global dental technology advances in 2026, procurement decisions must account for compatibility, serviceability, and long-term reliability. Below are five critical questions dental clinics and distributors should ask when sourcing equipment from the largest dental equipment manufacturers.
1. What voltage and electrical specifications do your dental units and imaging systems require, and are they compatible with international grid standards?
Why it matters: The largest manufacturers—including A-dec, Planmeca, and Belmont—offer equipment designed for regional electrical standards (e.g., 110–120V for North America, 220–240V for EU/Asia). Always confirm whether units are factory-configured for your local supply voltage or if step-down transformers are needed. In 2026, many premium models support dual voltage or come with modular power supplies to ease global deployment, especially for multi-location clinics and distributors.
2. What is your global spare parts availability policy, and how quickly can replacement components be delivered?
Why it matters: Leading manufacturers such as Dentsply Sirona and Envista maintain regional distribution hubs to ensure spare parts (e.g., handpiece motors, chair control valves, sensor cables) are dispatched within 24–72 hours. Distributors should verify SLAs (Service Level Agreements) for part fulfillment and confirm whether critical components are stocked locally. In 2026, OEMs increasingly use predictive analytics to pre-position high-failure-rate parts in key markets.
3. Do you provide on-site installation and commissioning services, and are they included in the purchase price?
Why it matters: High-end dental equipment (e.g., CBCT scanners, integrated operatory systems) requires certified technicians for proper installation, calibration, and integration with clinic IT infrastructure. Top manufacturers typically offer turnkey installation as part of enterprise contracts. Confirm whether services include plumbing, electrical tie-ins, DICOM configuration, and staff training—especially important for distributors managing client rollouts across multiple regions.
4. What is the standard warranty coverage for equipment, and are extended service contracts available?
Why it matters: In 2026, most major manufacturers provide a 2-year comprehensive warranty covering parts, labor, and software updates. Extended warranties (up to 5 years) are available and recommended for imaging systems and CAD/CAM units. Distributors should understand warranty transferability, especially for resale or clinic relocation. Note: Some OEMs now offer cloud-based warranty tracking and automated service alerts via IoT-enabled devices.
5. How are firmware updates and software maintenance handled under warranty and post-warranty periods?
Why it matters: Modern dental systems rely on integrated software for imaging, patient management, and diagnostics. Leading manufacturers provide over-the-air (OTA) firmware updates during the warranty term at no cost. Post-warranty, clinics and distributors must evaluate subscription models for continued software support, cybersecurity patches, and feature upgrades—particularly relevant for compliance with evolving data protection regulations (e.g., GDPR, HIPAA).
Need a Quote for Largest Dental Equipment Manufacturers?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


