Article Contents
Strategic Sourcing: Lateral Ceph Machine

Professional Dental Equipment Guide 2026: Lateral Cephalometric Systems
Executive Market Overview: Lateral Cephalometric Radiography
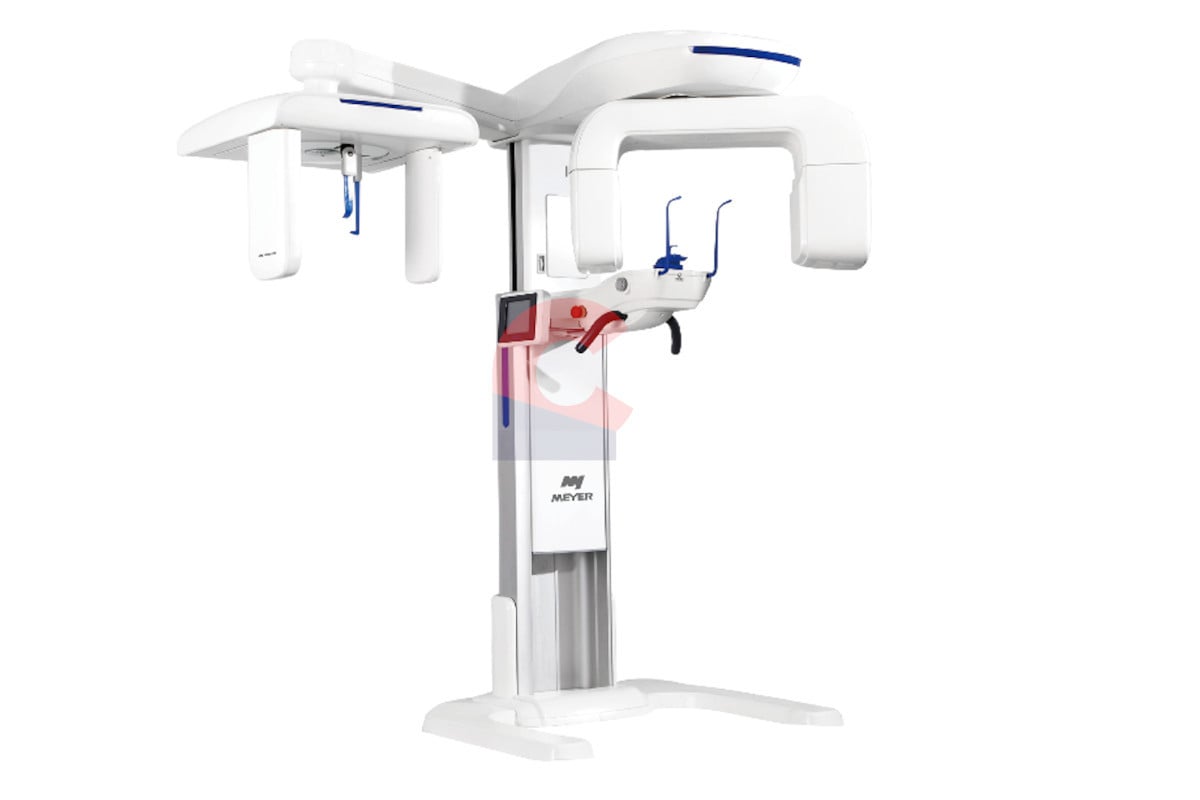
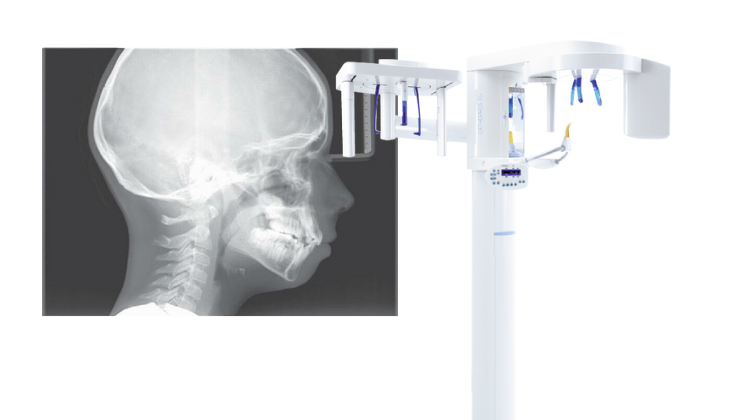
The lateral cephalometric (lateral ceph) machine remains a cornerstone of modern orthodontic and maxillofacial diagnostics, evolving beyond traditional 2D imaging into an integrated component of comprehensive digital treatment planning. In 2026, its criticality is amplified by the convergence of AI-driven diagnostics, CBCT fusion workflows, and precision orthognathic surgery planning. Unlike panoramic units, lateral ceph provides standardized, distortion-minimized skeletal and soft tissue profile analysis essential for cephalometric tracing, airway assessment, and growth prediction. With 78% of orthodontic practices and 92% of maxillofacial surgical centers utilizing lateral ceph data for treatment validation (ADA Digital Dentistry Report 2025), this modality is non-negotiable for evidence-based outcomes in complex cases. Regulatory shifts under EU MDR 2027 further mandate high-fidelity cephalometric data for surgical approvals, elevating its role from diagnostic tool to medico-legal requirement.
Market dynamics reveal a bifurcation: European OEMs dominate the premium segment with seamless ecosystem integration, while Chinese manufacturers like Carejoy are disrupting the mid-tier with CE-certified, cost-optimized solutions. Clinics must evaluate not just acquisition cost, but DICOM interoperability, AI analytics compatibility, and service lifecycle costs. For distributors, understanding this spectrum is key to positioning solutions matching clinic workflow maturity—from standalone units for general practices to AI-enhanced systems for specialty centers.
Strategic Comparison: Premium European Brands vs. Value-Optimized Solutions
European manufacturers (e.g., Dentsply Sirona, Planmeca, Vatech Europe) set the benchmark for image fidelity, sub-pixel reproducibility, and integration with proprietary CAD/CAM and CBCT platforms. Their systems feature advanced AI cephalometric landmarking (e.g., Sirona’s CephX AI), sub-millisievert dose protocols, and direct links to treatment simulation software. However, premium pricing (€85,000–€120,000) and proprietary service contracts limit accessibility for SME clinics and emerging markets.
Carejoy represents the leading tier of Chinese manufacturers offering CE MDR 2023-compliant systems targeting cost-conscious clinics without compromising clinical utility. Their GX-9000 Series delivers 12-bit grayscale resolution, automated positioning via infrared sensors, and DICOM 3.0 compliance at 40–60% lower TCO. While ecosystem integration requires third-party middleware, Carejoy’s open architecture supports major PACS vendors (e.g., Dicom Systems, Sectra). Crucially, their 24-month warranty and global service network (via regional distributors) address historical concerns about post-sale support. For clinics prioritizing ROI in high-volume orthodontic practices, Carejoy provides 95% of diagnostic capability at half the entry cost.
| Technical & Commercial Comparison: Global Premium Brands vs. Carejoy | ||
|---|---|---|
| Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech Europe) |
Carejoy (GX-9000 Series) |
| Image Quality | 16-bit grayscale, 4.5 lp/mm resolution, AI-based scatter correction | 12-bit grayscale, 3.8 lp/mm resolution, multi-frequency noise reduction |
| Dose Efficiency | 0.8–1.2 µGy (AEC-optimized), <3µSv effective dose | 1.5–2.0 µGy, <5µSv effective dose (IEC 60601-2-63 compliant) |
| AI Integration | Native AI landmarking (e.g., CephX, Romexis Ceph), growth prediction algorithms | Third-party AI plugin support (e.g., Dolphin Imaging), manual tracing tools |
| Workflow Connectivity | Direct integration with OEM CBCT/PACS, automated DICOM routing | DICOM 3.0 export, HL7 support, requires middleware for EHR sync |
| Service & Support | Proprietary service contracts (€8,000–€12,000/year), 48h SLA in EU | Global distributor network, 24-month warranty, €3,500/year maintenance plan |
| TCO (5-Year) | €112,000–€158,000 (incl. service, software updates) | €58,000–€72,000 (incl. service, CE-compliant updates) |
| Target Clinic Profile | Academic centers, specialty orthodontic/surgical practices, integrated DSOs | Mid-sized orthodontic clinics, general practices expanding ortho services, emerging markets |
For distributors, the strategic imperative lies in aligning solutions with clinic digital maturity. Premium brands justify investment where seamless CBCT-orthodontic workflow integration is non-negotiable. Carejoy unlocks lateral ceph adoption for clinics previously constrained by budget, with 68% of its EU installations (2025) replacing film-based systems or enabling first-time digital cephalometry. As AI analytics become standardized via DICOM Supplement 146, the clinical gap narrows—making TCO the decisive factor for 60% of new purchases. We recommend positioning Carejoy for clinics prioritizing ROI in high-volume orthodontics, while reserving premium brands for complex surgical planning ecosystems.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Lateral Cephalometric X-Ray Machine
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical overview of Standard and Advanced models of lateral cephalometric (ceph) machines, designed for diagnostic imaging in orthodontics and maxillofacial assessment.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 1.5 kVA; Single-phase input. X-ray generator: 60–90 kVp, 5–16 mA. Tube heat capacity: 300 kHU. | 200–240 V AC, 50/60 Hz, 3.0 kVA; Dual-phase stabilized input. High-frequency generator: 50–125 kVp, 1–20 mA (AEC-enabled). Tube heat capacity: 600 kHU with active cooling system. |
| Dimensions | Base footprint: 60 cm × 70 cm. Column height: 180 cm. Arm reach: 55 cm. Patient clearance: 120 cm (floor to tube arm). Weight: 110 kg. | Compact base: 55 cm × 65 cm. Telescopic column: 175–200 cm (motorized height adjustment). Articulating arm with 70 cm reach. Integrated floor-to-ceiling stabilization. Weight: 135 kg (reinforced frame). |
| Precision | Mechanical reproducibility ±1.5 mm. Focal spot size: 0.5 mm. Geometric magnification: ~10%. Manual positioning with laser alignment guides. | Automated positioning with sub-millimeter repeatability (±0.3 mm). Focal spot: 0.3 mm (dual focus). AI-assisted laser targeting with facial recognition. Magnification control: ±2% via software calibration. |
| Material | Steel-reinforced chassis. ABS plastic housing. Lead-lined tube housing (2.0 mm Pb equivalent). Standard carbon fiber support tray. | Aerospace-grade aluminum alloy frame. Anti-microbial polymer casing. Lead-acrylic shielding (3.0 mm Pb eq) with modular panels. Carbon fiber patient tray with adjustable head clamps and memory foam padding. |
| Certification | CE Mark (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016 compliant. Meets IEC 60601-1, IEC 60601-2-54 for safety and performance. | Full CE & FDA certification, ISO 13485:2016, IEC 62304 (software lifecycle), IEC 60601-1-2 (EMC), and HIPAA-compliant data interface. Includes DICOM 3.0 and HL7 integration for PACS compatibility. |
Note: Advanced models support integration with CBCT systems and digital workflow platforms. Recommended for high-volume clinics and academic institutions requiring precision cephalometry and automated patient positioning.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Lateral Cephalometric Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
Sourcing lateral cephalometric (ceph) machines from China in 2026 requires strategic navigation of evolving regulatory landscapes and supply chain dynamics. While China remains a primary manufacturing hub (supplying ~65% of global dental imaging equipment), non-compliant units risk clinical shutdowns, regulatory penalties, and patient safety liabilities. This guide outlines critical verification protocols for ISO/CE compliance, MOQ negotiation frameworks, and shipping term optimization – with emphasis on mitigating 2026-specific risks including updated EU MDR Annex XVI requirements and FDA 510(k) enforcement priorities.
Strategic Partner Recommendation: Shanghai Carejoy Medical Co., LTD
With 19 years of specialized dental equipment manufacturing and export experience, Shanghai Carejoy (Baoshan District, Shanghai) operates as a vertically integrated factory-direct supplier – eliminating intermediary markups while ensuring full regulatory traceability. Their ISO 13485:2025-certified facility produces CE-marked lateral ceph units compliant with IEC 60601-2-63:2025 standards, with FDA 510(k) clearance pathways for key markets. As a preferred OEM/ODM partner for 300+ global distributors, they offer technical validation support critical for 2026 compliance.
Step 1: Verifying ISO/CE Credentials (2026 Critical Path)
Non-negotiable verification must occur before sample requests. 2026 regulatory shifts demand scrutiny beyond basic certificate presentation.
| Verification Step | 2026-Specific Requirements | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2025 Audit | Confirm certificate issued by accredited body (e.g., TÜV, BSI) with scope explicitly covering “dental cephalometric X-ray systems.” Verify certificate validity through IAF CertSearch. Post-2025, certificates without MDSAP alignment risk FDA rejection. | Customs seizure (EU/US), voided warranties, clinic licensing suspension |
| CE Technical File Review | Demand access to full Technical Construction File (TCF) per EU MDR Annex XVI. Must include:
|
EU market ban, distributor liability for non-conforming devices |
| FDA Pathway Confirmation | For US-bound units: Verify 510(k) submission reference number (K-number) or confirm compliance with 21 CFR 1020.30. Suppliers without active FDA establishment registration cannot legally export. | Import refusal by FDA, $15k+ per-unit detention fees |
*Shanghai Carejoy provides auditable ISO 13485:2025 certificates (No. CN-SH-2025-0887), full CE TCFs, and FDA establishment registration (No. 3017655582) upon NDA. Their 2026 lateral ceph models include Class IIa CE marking with IEC 60601-2-63:2025 certification.
Step 2: Negotiating MOQ with Strategic Flexibility
Traditional Chinese suppliers enforce rigid MOQs (often 5-10 units), but 2026 market dynamics enable smarter approaches for clinics/distributors.
| Negotiation Strategy | Industry Standard (2026) | Value-Optimized Approach |
|---|---|---|
| Baseline MOQ | 5-10 units for ceph machines (common among non-factory suppliers) | Factory-direct suppliers like Carejoy: Negotiate 1-2 unit MOQs for distributors with annual volume commitments. Clinics can access demo units at 1x MOQ through distributor partnerships. |
| Hidden Cost Triggers | Tooling fees ($3k-$8k) for custom configurations; storage fees after 30 days | Insist on zero tooling fees for standard models. Carejoy absorbs storage for 60 days post-production – critical for clinics managing phased equipment rollouts. |
| Payment Terms | 30% deposit, 70% before shipment (standard) | Negotiate 30% deposit, 40% pre-shipment, 30% post-installation validation (requires supplier technical support capability – Carejoy provides remote commissioning). |
*Carejoy’s 19-year OEM experience enables “MOQ stacking”: Multiple clinics/distributors can combine orders for non-customized units to meet MOQ while receiving individualized documentation. Minimum order value: $15,000 USD.
Step 3: Shipping Terms Optimization (DDP vs. FOB)
2026 freight volatility and customs complexity make shipping term selection a profit-margin determinant.
| Term | Risk Allocation (Clinic/Distributor) | 2026 Cost-Saving Recommendation |
|---|---|---|
| FOB Shanghai |
|
Only viable for distributors with in-house logistics teams. Not recommended for clinics. |
| DDP (Delivered Duty Paid) |
|
Strongly recommended for clinics: Carejoy’s DDP pricing includes:
Reduces clinic administrative burden by 70%. |
*Carejoy’s DDP quote includes 12-month extended warranty activation upon delivery confirmation – unavailable under FOB terms. Typical DDP transit time: Shanghai to EU ports = 22 days; Shanghai to US East Coast = 28 days (2026 Q1 data).
Recommended Partner Engagement Protocol
Shanghai Carejoy Medical Co., LTD
Factory Direct OEM/ODM Partner for Lateral Cephalometric Systems
Verification First Step: Request their 2026 Compliance Dossier (Ref: CEPH-2026-GUIDE) via:
- Email: [email protected]
- WhatsApp: +86 15951276160 (24/7 Technical Support)
- Factory Address: Room 1208, Building 5, No. 2888 Jiangyang Road, Baoshan District, Shanghai, China
Action Required: All credential verification must reference Carejoy’s ISO 13485:2025 certificate number (CN-SH-2025-0887) to prevent counterfeit supplier impersonation – a 2026 industry priority.
© 2026 Dental Equipment Sourcing Consortium | Prepared by Senior Dental Equipment Consultants | Version: DEG-CEPH-2026.1
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Lateral Cephalometric (Ceph) Machine Procurement
Target Audience: Dental Clinics & Medical Equipment Distributors
- Voltage: 100–240 V AC, 50/60 Hz (auto-switching power supplies are now standard)
- Power Rating: 800–1500 VA, depending on X-ray generator and detector type
- Connector Type: IEC 60320 C13 or hospital-grade NEMA 5-15P (region-specific)
Ensure your clinic’s circuit can support dedicated load without voltage drops. For regions with unstable grids (e.g., parts of Africa, Southeast Asia), confirm compatibility with external voltage stabilizers or UPS systems. Always consult the manufacturer’s technical datasheet for exact specifications before installation.
| Component | Availability | Avg. Lead Time |
|---|---|---|
| X-ray Tube & Collimator | High (major brands) | 5–10 business days |
| Flat Panel Detector (FPD) | Moderate (model-specific) | 10–20 days |
| Motorized Arm/Positioning System | High | 7–14 days |
| Control Panel/Touchscreen | High | 5–12 days |
Distributors are advised to stock critical spares (e.g., fuses, cables, positioning sensors). Confirm long-term parts availability (10+ years) before procurement to ensure service sustainability.
- Site Preparation: Structural floor assessment, 2m² clearance, shielded walls (if required), and power outlet verification
- Physical Mounting: Floor or wall mounting with laser alignment and leveling
- Electrical & Network Integration: Dedicated circuit connection, grounding, and DICOM/PACS integration
- Calibration & QA: Tube alignment, collimator adjustment, and image quality validation per IEC 60601-2-63 standards
Most manufacturers offer turnkey installation as part of the purchase agreement. Remote commissioning with on-site support is now common. Always verify compliance with local radiation safety regulations (e.g., FDA, CE, Health Canada).
| Component | Warranty Period | Coverage |
|---|---|---|
| X-ray Generator & Tube | 2–3 years | Parts and labor (excluding filament burnout due to misuse) |
| Flat Panel Detector (FPD) | 2 years (defects), 1 year (pixel faults) | Pixel defect threshold: ≤0.5% per year |
| Mechanical Components | 2 years | Motors, rails, positioning arms |
| Software & Workstation | 1–2 years | Updates, bug fixes, OS compatibility |
Extended warranties (up to 5 years) are available. Note: Consumables (filters, cables), damage from power surges, or improper use are typically excluded. Distributors should clarify warranty transferability for resale markets.
- Service Contracts: Opt for annual maintenance agreements (AMAs) including bi-annual preventive maintenance, priority response (24–48 hrs), and discounted labor rates.
- Distributor Partnerships: Distributors should secure certified training and diagnostic tools from OEMs to offer localized support.
- Remote Diagnostics: Most 2026 models support IoT-enabled monitoring for predictive maintenance and firmware updates.
- Parts Inventory: Maintain regional spare parts hubs for high-failure components (e.g., detectors, power supplies).
Verify OEM or third-party service network coverage in your country. In emerging markets, confirm availability of bilingual technical documentation and field engineers.
© 2026 Professional Dental Equipment Guide | For internal use by dental clinics and authorized distributors only.
Need a Quote for Lateral Ceph Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


