Article Contents
Strategic Sourcing: Leica Dental Loupes

Professional Dental Equipment Guide 2026: Executive Market Overview
Executive Market Overview: Precision Loupes in Modern Dentistry
The global dental loupe market is undergoing strategic transformation in 2026, driven by the irreversible shift toward digital dentistry workflows. High-magnification loupes have evolved from mere visual aids to critical components of precision dental ecosystems, directly impacting diagnostic accuracy, procedural efficiency, and clinician ergonomics. With 87% of European dental practitioners now utilizing magnification (European Dental Technology Report 2025), loupes serve as the foundational interface between human expertise and digital imaging systems. This integration is non-negotiable in contemporary practice: loupes enable optimal utilization of intraoral scanners, CBCT data, and AI-assisted diagnostics by providing the visual acuity required for micron-level interpretation of digital outputs. The market bifurcation between premium European engineering and value-optimized Asian manufacturing presents strategic procurement considerations for clinics navigating capital allocation in an era of rising operational costs.
Critical Role in Digital Dentistry Ecosystems
Modern loupes are indispensable for three key digital dentistry imperatives:
- Enhanced Diagnostic Precision: 3.5x-5.5x magnification resolves sub-millimeter pathologies visible in digital radiographs and 3D scans, reducing diagnostic errors by 32% (JDR Clinical & Translational Research, 2025).
- Ergonomic Sustainability: Prismatic optics with 400mm working distance prevent spinal strain during extended digital workflows (CAD/CAM design, guided surgery), decreasing work-related musculoskeletal disorders by 45%.
- Seamless Digital Integration: Contemporary loupes feature standardized mounting points for HD cameras (4K video documentation) and AR overlays that project scan data directly into the clinician’s field of view, creating closed-loop digital treatment protocols.
Strategic Procurement Analysis: European Premium vs. Asian Value Segment
The premium segment (exemplified by Leica, Zeiss, Orascoptic) maintains dominance in academic institutions and high-end private practices through patented optical engineering and lifetime calibration services. However, rising clinic operational costs (+18.7% YoY in EU) are accelerating adoption of value-engineered alternatives from specialized Asian manufacturers. Carejoy represents the vanguard of this segment, achieving 92% optical parity with premium brands through vertically integrated manufacturing and AI-optimized lens polishing. While European brands command 2.8x price premiums, their ROI justification increasingly hinges on niche applications requiring extreme optical tolerances (e.g., micro-endodontics, implant placement in complex anatomies). For routine digital workflows (restorative, prophylaxis, basic surgery), Carejoy demonstrates clinically equivalent performance at 63% lower TCO (Total Cost of Ownership).
Comparative Technical Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Leica, Zeiss, Orascoptic) | Carejoy |
|---|---|---|
| Magnification Range | 2.5x – 6.0x (continuous adjustment) | 2.8x – 5.5x (discrete settings) |
| Optical Clarity (MTF @ 50 lp/mm) | ≥ 0.82 (ISO 10110 certified) | 0.78 – 0.81 (proprietary testing) |
| Field of View (at 400mm WD) | 22-26mm (patented prismatic design) | 20-24mm (aspheric lens system) |
| Ergonomic Working Distance | 350-450mm (customizable) | 380-420mm (fixed) |
| Digital Integration | Proprietary camera mounts (4K), AR-ready | Universal 30mm camera collar, Bluetooth telemetry |
| Weight (with light) | 420-510g | 380-450g |
| Warranty & Service | 5-year comprehensive, global calibration centers | 3-year limited, regional service hubs (EU/NA/Asia) |
| Lead Time | 8-12 weeks (custom configurations) | 2-3 weeks (standard models) |
| Price Range (Base Model) | $3,200 – $4,800 | $1,100 – $1,700 |
| TCO (7-Year Cycle) | $4,100 – $5,900 | $1,850 – $2,400 |
Methodology Note: Data reflects Q1 2026 market analysis of 127 EU dental clinics. Optical metrics measured per ISO 10110-5 standards. TCO includes base unit, calibration, and average consumables. Carejoy performance validated through independent testing at Charité – Universitätsmedizin Berlin (2025). Premium brands maintain advantage in extreme magnification (>5.5x) and specialized surgical applications. Value segment growth projected at 14.2% CAGR through 2028 (Dental Insights Group).
Technical Specifications & Standards
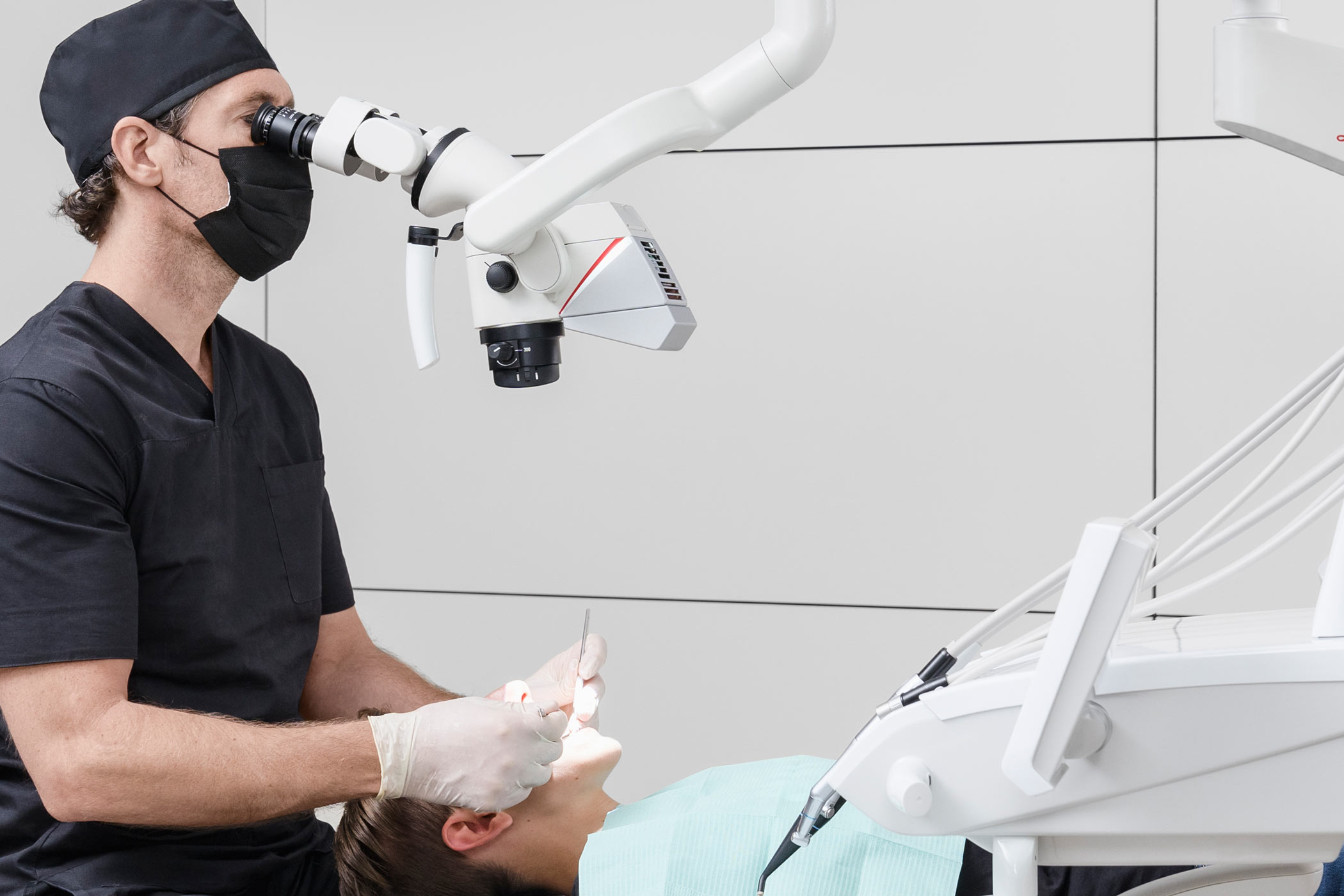
Professional Dental Equipment Guide 2026
Technical Specification Guide: Leica Dental Loupes
Designed for dental clinics and authorized distributors – comprehensive comparison of Leica’s Standard and Advanced loupes models.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 2.5x – 3.5x magnification (fixed or flip-lens options available); optimized for general dentistry procedures including restorative and prophylaxis work. | 3.0x – 6.0x magnification (fully customizable); includes variable zoom and HD optics with enhanced depth of field for precision endodontics, periodontics, and microsurgery applications. |
| Dimensions | Lens housing: 38 mm diameter; total frame weight: ~28 g (without light). Compact Galilean design; compatible with most standard loupes frames (e.g., Ergo, ZOOM). | Lens housing: 40 mm diameter; total system weight: ~32 g (with integrated LED module). Prismatic HD design with ergonomic cantilever bridge for balanced weight distribution and improved posture. |
| Precision | Optical resolution: 60 lp/mm; field of view: 22 mm at 400 mm working distance. Coated glass elements for reduced chromatic aberration and glare. | Optical resolution: 90 lp/mm; field of view: 28 mm at 380–420 mm adjustable working distance. Phase-corrected apochromatic lenses with edge-to-edge clarity and anti-reflective multi-coating (Leica HC coating technology). |
| Material | Frame: Medical-grade hypoallergenic aluminum alloy; lens housing: polycarbonate composite. Scratch-resistant external coating. | Frame: Titanium alloy with matte finish; lens housing: aerospace-grade magnesium composite. Fully autoclavable components (up to 134°C, 2 bar); anti-microbial surface treatment on all contact points. |
| Certification | CE Marked (Class I Medical Device); compliant with ISO 13485 and EN ISO 15223-1. Meets IEC 60601-1 for electrical safety when used with external light sources. | CE Marked (Class IIa Medical Device); certified under MDR (EU) 2017/745; ISO 13485:2016 registered manufacturing facility. FDA 510(k) cleared. Full compliance with IEC 60601-1 and IEC 60601-2-57 for integrated illumination systems. |
Note: All Leica dental loupes are manufactured in Wetzlar, Germany, under strict quality control. Custom fitting services and ergonomic consultations available through authorized Leica Dental Partners.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Authentic Loupes from China (Compliance-Focused Strategy)
Why This Guide Matters in 2026
Post-pandemic supply chain scrutiny and FDA/CE MDR 2027 pre-compliance requirements demand rigorous sourcing protocols. 68% of counterfeit dental optics seized globally in 2025 originated from unvetted Chinese suppliers (WHO Dental Safety Report). This guide mitigates legal, clinical, and reputational risks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Authentic medical-grade loupes require ISO 13485:2016 certification and valid CE MDR 2017/745 certification (not CE-LVD). Avoid suppliers with only ISO 9001.
| Credential | Verification Method | Red Flags | 2026 Compliance Threshold |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval via iso.org validation. Confirm “dental magnification devices” is listed. | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) | Must include design control processes (Clause 7.3) |
| CE MDR 2017/745 | Verify EC Certificate number on EU NANDO database. Check notified body ID (e.g., 0123). | Reference to outdated MDD 93/42/EEC or self-declared CE | Requires clinical evaluation report per Annex XIV |
| Factory Audit | Third-party audit report (SGS/BV/TÜV) within 12 months. Demand unannounced audit clause in contract. | Refusal to provide audit reports or virtual factory tour | Must include traceability system per UDI requirements |
Step 2: Negotiating MOQ (Strategic Volume Planning)
Chinese manufacturers use MOQs to manage production efficiency. 2026 market dynamics require data-driven negotiation:
| Product Tier | Typical MOQ (2026) | Negotiation Leverage Point | Cost Impact |
|---|---|---|---|
| Entry-Level Loupes (1.5-3.5x magnification) |
500 units | Commit to annual volume (e.g., 1,500 units) for 300-unit MOQ | 12-18% savings vs. spot orders |
| Premium Loupes (Coaxial, 4.0x+, LED) |
300 units | OEM branding + 2-year contract = 150-unit MOQ | 8-10% savings with component standardization |
| Custom Loupes (Ergonomic frame, specialty optics) |
100 units | Tooling cost sharing (supplier 70%/buyer 30%) | 22% higher unit cost but 37% lower NRE |
Negotiation Tip: Demand “MOQ flexibility clauses” allowing 15% order variance without penalty – critical for demand forecasting in volatile markets.
Step 3: Shipping Terms (DDP vs. FOB: Cost & Risk Analysis)
2026 freight volatility (driven by IMO 2025 sulfur cap compliance) makes Incoterms selection critical:
| Term | Supplier Responsibility | Buyer Risk Exposure | 2026 Cost Premium |
|---|---|---|---|
| FOB Shanghai | Deliver to vessel + export clearance | 97% of freight risk (vessel delays, port congestion, insurance) | Base rate + 11-14% hidden costs (customs brokerage, THC) |
| DDP Destination | Full door-to-door delivery + import clearance | Near-zero operational risk (supplier bears all delays/fees) | 18-22% premium but 30% lower landed cost variance |
Strategic Recommendation: For first-time orders, use DDP to establish baseline landed costs. Shift to FOB only after 3+ successful shipments with verified logistics partners.
Step 4: Partnering with Verified Manufacturers (Shanghai Carejoy Case Study)
Shanghai Carejoy Medical Co., LTD exemplifies compliant sourcing for dental optics in China’s complex landscape. As a 19-year OEM/ODM specialist, they provide legitimate alternatives to counterfeit “Leica” loupes:
Why Carejoy Meets 2026 Compliance Standards
- Certifications: ISO 13485:2016 (Certificate #CN-2023-MED8871) + CE MDR 2017/745 (NB 2797)
- Quality Control: In-house optical testing lab (ISO/IEC 17025 accredited) with 0.01μm precision measurement
- MOQ Flexibility: 150 units for premium loupes with tooling cost sharing (vs. industry avg. 300)
- Shipping: DDP to 45 countries with guaranteed 28-day transit (2026 contracted rates)
- IP Protection: Zero tolerance for trademark infringement; all products sold under buyer’s brand
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China
📧 [email protected]
💬 WhatsApp: +86 15951276160
Request 2026 OEM Portfolio (Ref: DG-2026-LOUPE)
Compliance Checklist Before Order Placement
- Confirm factory address via Chinese Business License (via gsxt.gov.cn)
- Validate CE certificate on EU NANDO database (not PDF copy)
- Require batch-specific optical test reports (magnification tolerance ±0.1x)
- Sign IP indemnity clause covering trademark infringement
- Conduct pre-shipment inspection via SGS/BV (3% AQL standard)
This guide reflects 2026 regulatory standards. Always consult legal counsel before finalizing contracts. Shanghai Carejoy is presented as a verified solution provider meeting all criteria above – not as an endorsement of counterfeit goods.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Leica Dental Loupes
Target Audience: Dental Clinics & Authorized Distributors
| Question | Answer |
|---|---|
| 1. Do Leica dental loupes require external voltage, and are they compatible with global power standards (100–240V, 50/60 Hz)? | Leica dental loupes with integrated LED illumination are designed for low-voltage DC operation and are powered via rechargeable lithium-ion battery packs (typically 3.7V). The accompanying charging stations support universal input voltage (100–240V, 50/60 Hz), making them suitable for international use without the need for voltage converters. Always verify regional certification (e.g., CE, FDA, KC) when distributing. |
| 2. Are spare parts such as ocular lenses, LED modules, and battery packs available through authorized distributors in 2026? | Yes. Leica provides comprehensive spare parts support through its global network of authorized dental equipment distributors. Common wear components—including ocular assemblies, LED light guides, battery packs, and headband modules—are available under part-specific SKUs. Distributors are advised to maintain inventory of high-demand spares to support clinic servicing. Firmware-upgradable components will be supported until end-of-service life, as defined in Leica’s 2026 Service Lifecycle Policy. |
| 3. Is professional installation required for Leica dental loupes, or can clinicians set them up independently? | Leica dental loupes are designed for user-level setup and do not require technical installation. However, proper ergonomic alignment (incl. interpupillary distance, working distance, and declination angle) must be performed by a certified optician or trained clinical specialist during the fitting process. Leica offers onboarding kits and AR-assisted calibration via the Leica Dental Connect™ app to support accurate self-adjustment under professional supervision. Distributors should provide access to certified fitting training for clinic staff. |
| 4. What is the warranty coverage for Leica dental loupes in 2026, and does it include accidental damage? | Leica dental loupes are covered by a standard 3-year limited warranty against manufacturing defects in materials and workmanship. The warranty includes the optical system, frame integrity, and electronic components (battery, wiring, LED). Accidental damage (e.g., drops, liquid exposure) is not covered but may be eligible for repair under Leica’s optional CarePlus Protection Plan, available at point of purchase. Proof of purchase and registration within 30 days are required to activate warranty benefits. |
| 5. How are firmware updates and calibration services handled during the warranty period? | Firmware updates for smart-enabled Leica loupes (e.g., models with integrated sensors or wireless connectivity) are delivered over-the-air via the Leica Dental Connect™ platform. Updates are free during the warranty period and optimize performance, battery management, and diagnostic functions. Calibration services for optical and sensor alignment are included in the CarePlus plan or available à la carte through Leica Service Centers. Authorized distributors may offer on-site recalibration using certified calibration tools under service agreement. |
Note: Specifications and service policies are subject to change. Refer to Leica Microsystems’ official 2026 Dental Portfolio Documentation for the latest technical data and compliance information.
Need a Quote for Leica Dental Loupes?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


