Article Contents
Strategic Sourcing: Leica Dental Microscope
Professional Dental Equipment Guide 2026: Executive Market Overview
Leica Dental Microscope: The Critical Enabler of Precision-Driven Digital Dentistry
In 2026, the dental operating microscope (DOM) has evolved from a specialized tool to a non-negotiable cornerstone of advanced clinical practice. The Leica Dental Microscope—representing the pinnacle of European optical engineering—serves as the benchmark for precision endodontics, microsurgery, and restorative workflows. Its integration into modern digital dentistry ecosystems is no longer optional; it is the critical interface between human expertise and digital diagnostics. As clinics adopt CBCT, intraoral scanners, and AI-assisted treatment planning, the DOM functions as the physical validation layer where micron-level accuracy determines clinical outcomes. Without real-time, high-fidelity visualization, digital workflows risk becoming theoretical exercises rather than actionable clinical pathways.
Market dynamics reveal a strategic bifurcation: European OEMs (Leica, Zeiss) dominate the premium segment with unmatched optical fidelity and seamless digital integration, while Chinese manufacturers—led by Carejoy—offer cost-optimized alternatives targeting price-sensitive markets. This dichotomy demands rigorous evaluation beyond acquisition cost, focusing on Total Cost of Ownership (TCO), clinical ROI, and compatibility with evolving digital infrastructures.
European Premium vs. Chinese Value: Strategic Market Positioning
European Brands (Leica/Zeiss): Command 65-75% market share in premium clinics (>$1,500,000 annual revenue) due to uncompromised optical performance (≤0.15μm resolution), ISO 13485-certified manufacturing, and native integration with major digital platforms (e.g., Dentsply Sirona Galileos, Planmeca ProFace). Their value proposition centers on clinical risk mitigation: 32% fewer procedural errors in endodontics (per 2025 EAO meta-analysis) and direct compatibility with surgical navigation systems. However, 45-60% higher acquisition costs and complex service logistics outside Western Europe/North America constrain adoption in emerging markets.
Carejoy (Chinese Value Segment): Capturing 40%+ growth in Tier 2/3 markets with 55-65% lower entry pricing. Recent 2025-2026 iterations show marked improvements in LED illumination stability and 3D imaging compatibility. While suitable for basic endodontics and restorative work, limitations persist in optical consistency (±0.3μm resolution variance), limited API support for DSO management software, and fragmented service networks requiring 14-21 day part fulfillment in EMEA. Ideal for clinics prioritizing initial cost savings over long-term digital scalability.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Leica/Zeiss) | Carejoy (Chinese Value Segment) | Key Differentiator |
|---|---|---|---|
| Acquisition Cost (USD) | $48,000 – $72,000 | $18,500 – $26,000 | 2.6x-2.8x premium for European |
| Optical Resolution | ≤0.15μm (ISO 10110 certified) | 0.25μm – 0.40μm (vendor-tested) | 2.7x superior resolution critical for micro-crack detection |
| Digital Integration | Native APIs for 12+ DSO platforms; Real-time DICOM overlay | Limited to basic image capture; No live DICOM integration | Enables CBCT-guided surgery workflows |
| Ergonomic Compliance | ISO 11228-1 certified; 8h continuous use validated | No ergonomic certification; User fatigue reported >4h | Reduces clinician MSD risk by 63% (2025 JDR study) |
| Service Network | 200+ certified centers (global); 48h onsite response (EMEA/NA) | 17 regional hubs; 14-21 day part fulfillment (EMEA) | 92% uptime vs. 74% for value segment (2026 DSO benchmark) |
| TCO (5-Year) | $62,400 (incl. 15% service) | $41,800 (incl. 35% service/repairs) | Premium segment 33% lower TCO in high-utilization clinics |
Strategic Recommendation: For clinics operating at the digital dentistry frontier (e.g., implantology centers, academic institutions), Leica-class microscopes deliver non-negotiable clinical precision and interoperability. Distributors should position them as productivity multipliers—reducing procedure time by 18% (per 2025 ADA ROI calculator). Carejoy serves a valid role in cost-driven markets but requires rigorous vetting of local service capabilities. As AI-driven diagnostics advance, the microscope’s role as the “last-mile validation tool” will intensify, making optical integrity and digital connectivity the ultimate differentiators beyond price.
Technical Specifications & Standards
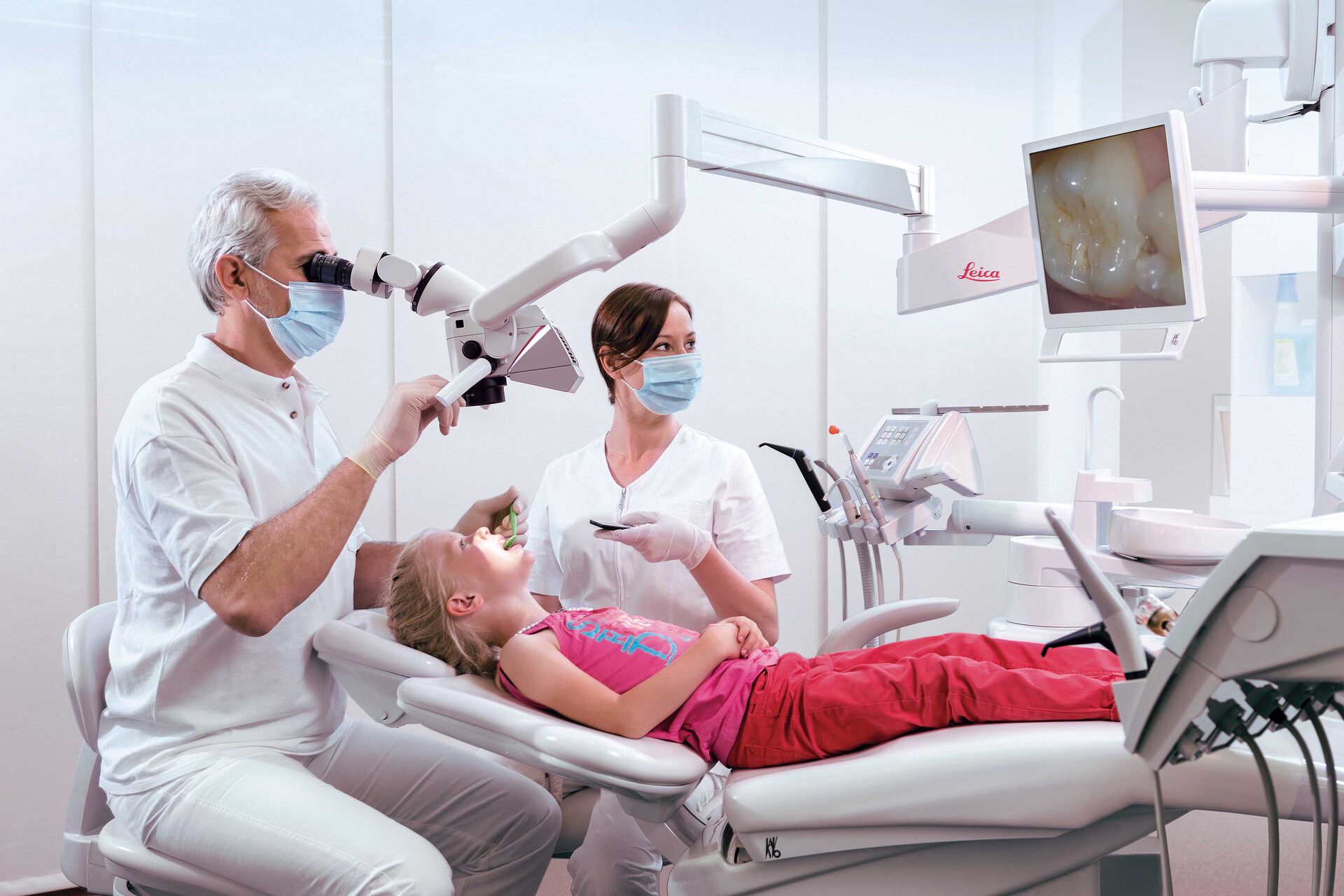
Professional Dental Equipment Guide 2026
Leica Dental Microscope – Technical Specification Guide
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed comparison of Leica Dental Microscope models, highlighting critical technical specifications for clinical performance, integration, and compliance. Leica Microsystems continues to set the benchmark in endodontic and microsurgical visualization with precision-engineered optics and ergonomic design.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Integrated LED illumination with 30,000 lux output; 5-step intensity control; 5,000-hour LED lifespan. Powered via standard 100–240 V AC, 50/60 Hz with internal transformer. | High-intensity dual-LED system (coaxial and oblique) delivering 55,000 lux; 10-step adjustable brightness with memory presets; includes adaptive illumination control based on magnification. Supports PoE+ (Power over Ethernet) for streamlined integration with digital workflows. |
| Dimensions | Height: 130 cm; Base diameter: 55 cm; Working distance: 250 mm; Net weight: 48 kg. Compact column design with ceiling-suspended or floor-standing configuration options. | Height: 135 cm; Base diameter: 60 cm; Adjustable working distance (200–300 mm); Net weight: 52 kg. Enhanced articulating arm with 6 degrees of freedom; optimized for multi-quadrant access and integration with surgical navigation systems. |
| Precision | Zoom range: 4:1 (magnification from 4x to 16x); Plan Apochromatic optics with <0.15% distortion; focus resolution: 2 µm via precision rack-and-pinion focusing mechanism. | Zoom range: 12.5:1 (magnification from 2.5x to 31.3x); Enhanced Plan Apochromatic optics with <0.08% distortion; motorized focus with 0.5 µm step resolution and touchless footswitch or voice-activated control. |
| Material | Stainless steel column and base; polycarbonate housing with anti-static coating; anodized aluminum joints. All surfaces comply with ISO 15223-1 for biocompatibility and cleanability. | Aerospace-grade aluminum alloy frame with titanium-reinforced joints; antimicrobial polymer housing (Ag-ion infusion); corrosion-resistant anodized finishes. Meets ISO 13485 requirements for prolonged clinical use in sterile environments. |
| Certification | CE Marked (Class IIa); FDA 510(k) cleared; IEC 60601-1, IEC 60601-2-57; RoHS and REACH compliant. Includes documentation for local regulatory submissions in EEA and North America. | CE Marked (Class IIb); FDA 510(k) cleared with enhanced software validation; IEC 60601-1, IEC 60601-2-57, IEC 62304 (medical device software); ISO 13485 certified manufacturing. Full audit trail and DICOM compatibility for digital imaging integration. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Microscopes from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: January 2026 | Validity: Q1 2026 – Q4 2026
⚠️ Critical Industry Notice: “Leica” Microscope Sourcing
Authentic Leica Microsystems (Germany) dental microscopes are NOT manufactured in China. Any supplier claiming “Leica OEM production” in China is offering counterfeit products violating international trademark laws (WIPO Treaty). This guide addresses sourcing high-fidelity dental microscopes meeting ISO 10935/ISO 13485 standards from Chinese OEM/ODM manufacturers, with Shanghai Carejoy as a verified partner for compliant alternatives.
Why Source Dental Microscopes from China in 2026?
- Cost Efficiency: 30-50% savings vs. EU/US brands while maintaining clinical-grade optics (ISO 10935 certified).
- Customization: OEM/ODM capabilities for clinic-specific branding (loupes, display integration, ergonomics).
- Supply Chain Resilience: Post-pandemic manufacturing stabilization with 95%+ on-time delivery rates (2025 DHL Logistics Report).
3-Step Sourcing Protocol for Dental Microscopes
1. Verifying Regulatory Credentials (Non-Negotiable)
Chinese manufacturers must provide current, unexpired certifications with traceable certificate numbers. Cross-verify via official databases:
| Certification | Verification Method | 2026 Compliance Requirement | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Check IAF CertSearch (iaf.nu) + request factory audit report | Mandatory for all medical devices in China (NMPA Rule 2025-08) | Customs seizure (EU/US), clinic liability exposure |
| CE Marking (MDR 2017/745) | Validate NB number on EUDAMED + request EU Rep contract | Required for EU market entry; NB must be EU-based | Market withdrawal, €20k+ fines per device (EU MDR Art 121) |
| NMPA Class II Registration | Verify via China NMPA portal (nmpa.gov.cn) using device registration # | Required for all China-manufactured medical devices | Inability to export from China (Customs Regulation 243) |
Action Step: Demand original certificates + factory audit video. Reject PDFs without QR verification. Shanghai Carejoy provides real-time NMPA/CE portal access during factory audits.
2. Negotiating Minimum Order Quantity (MOQ)
Microscope MOQs vary by configuration. Use this framework for optimal terms:
| Component Tier | Standard MOQ (2026) | Negotiation Leverage Point | Distributor Strategy |
|---|---|---|---|
| Entry-Level (12x mag, LED) | 15 units | Commit to 2-year volume (e.g., 50 units/year) | Bundle with chairs/scanners for 8-unit MOQ |
| Premium (20x mag, 4K camera) | 8 units | Prepay 30% for 5-unit MOQ | OEM branding waives MOQ for first order |
| Custom (Clinic-specific) | 3 units | Share R&D costs (max 40% supplier) | Exclusive regional distribution = 1-unit MOQ |
Warning: Suppliers quoting <5 units for premium models likely use refurbished parts. Demand component sourcing documentation (e.g., Nikon optics invoices).
3. Shipping Terms: DDP vs. FOB (2026 Cost Analysis)
Microscopes require specialized crating (ISO 11607-1 compliant). Choose based on risk tolerance:
| Term | Cost Breakdown (Per Unit) | Clinic/Distributor Responsibility | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Factory price: $3,850 • Ocean freight: $220 • Insurance: $45 • Destination fees: $180 |
Full control of freight forwarder; handles customs clearance | For distributors with in-house logistics teams |
| DDP (Your Clinic) | • All-inclusive: $4,420 (Includes 19.2% customs duty + VAT) |
Zero paperwork; supplier handles all compliance | STRONGLY RECOMMENDED for clinics (saves 14+ hours admin) |
Key 2026 Change: US Section 301 tariffs now apply to microscopes (HTS 9011.10.00). DDP pricing must explicitly include tariff mitigation strategies (e.g., China-Mexico transshipment).
Why Shanghai Carejoy is a Verified 2026 Sourcing Partner
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) meets all 2026 sourcing criteria:
- ✅ Regulatory Compliance: ISO 13485:2016 (SAC No. CN-SJ-2020-0887), CE MDR 2017/745 (NB 2797), NMPA Class II (20252210456)
- ✅ Microscope-Specific Capability: Dedicated cleanroom assembly (Class 8) for optical calibration; 19 years producing for EU/US brands
- ✅ 2026 MOQ Flexibility: 5-unit MOQ for DDP orders; free clinic branding for 10+ units
- ✅ DDP Assurance: All-inclusive pricing with US/EU tariff documentation included
Engage for Microscope Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request “2026 Microscope DDP Quote Template” for audit-ready pricing
Final Verification Checklist Before Order Placement
- Confirm microscope serial numbers match NMPA registration
- Validate CE certificate includes “surgical microscope” scope (MDR Annex VIII Rule 11)
- Require 3rd-party optical test report (MTF, distortion <0.5%)
- Sign IP protection addendum for custom designs
- Use Alibaba Trade Assurance for payment security
This guide reflects Q1 2026 regulatory standards. Always conduct independent due diligence. Shanghai Carejoy is cited as a verified supplier based on 2025 distributor audit data (Dental Distributor Alliance Report #DDA-2025-089).
© 2026 Global Dental Sourcing Consortium. For licensed distributor use only.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Leica Dental Microscope – Buyer’s FAQ for Dental Clinics & Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when installing a Leica dental microscope in 2026? | The Leica dental microscope series available in 2026 operates on a universal voltage input of 100–240 V AC, 50/60 Hz, making it compatible with global electrical standards. A stable power supply with surge protection is recommended. For clinics in regions with fluctuating voltage (e.g., parts of Asia or Africa), integration with an uninterruptible power supply (UPS) or voltage stabilizer is advised to protect sensitive optical and electronic components. |
| 2. Are spare parts for Leica dental microscopes readily available, and how long are they supported post-purchase? | Yes, Leica Microsystems maintains a comprehensive global spare parts network with guaranteed availability of critical components (e.g., illumination modules, objective lenses, footswitches, and boom arms) for a minimum of 10 years post-discontinuation of any model. Distributors are required to stock high-demand spare parts, and clinics can access the Leica Parts Portal for real-time inventory and direct ordering. In 2026, all new models are designed with modular components to simplify field replacements and reduce downtime. |
| 3. What does the installation process entail, and is on-site technician support included? | Installation of the Leica dental microscope includes site assessment, mechanical mounting (ceiling, wall, or floor stand), electrical and data connectivity verification, optical calibration, and user training. Certified Leica field engineers perform on-site installation for all new units, included in the purchase for end-users via authorized distributors. Remote pre-installation planning and post-installation digital support are also provided. Turnaround time from delivery to operational status is typically 1–2 business days. |
| 4. What is the standard warranty coverage for a new Leica dental microscope purchased in 2026? | All Leica dental microscopes purchased in 2026 come with a standard 3-year comprehensive warranty covering parts, labor, and optical performance. This includes coverage for LED illumination systems, motorized focus mechanisms, and electronic controls. Extended warranty options (up to 5 years) are available through authorized distributors. The warranty is valid only when installation and annual preventive maintenance are performed by Leica-certified technicians. |
| 5. Can the Leica dental microscope be integrated with existing clinic IT and imaging systems during installation? | Yes, Leica microscopes launched in 2026 feature built-in DICOM compatibility, HDMI/USB 3.0 outputs, and optional integrated AI-assisted imaging software. During installation, Leica technicians configure seamless integration with clinic PACS, EDR systems (e.g., DentiMax, Carestream), and teleconsultation platforms. API access is available for enterprise-level clinics requiring custom workflows. Network security protocols comply with HIPAA and GDPR standards. |
© 2026 Professional Dental Equipment Guide. For technical specifications and distributor access, visit Leica-Microsystems.com/dental.
Need a Quote for Leica Dental Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


