Article Contents
Strategic Sourcing: Leica Dental Microscope Price

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Microscopy: The Critical Catalyst for Precision Dentistry
In the rapidly evolving landscape of digital dentistry, the dental operating microscope (DOM) has transitioned from a specialty tool to an indispensable clinical cornerstone. Modern endodontic, prosthodontic, and microsurgical procedures demand magnification capabilities exceeding 4x-25x to achieve sub-millimeter precision – a threshold unattainable through loupes alone. The integration of DOMs with intraoral scanners, CBCT systems, and AI-driven diagnostic platforms has established them as the central nervous system of digitally integrated workflows. Clinics without DOM capabilities now face significant competitive disadvantages in complex case acceptance, treatment accuracy (reducing procedural errors by up to 37% per 2025 EAO studies), and patient outcomes documentation.
The Leica Dental Microscope (specifically the M530 series) remains the European gold standard, commanding premium positioning at €52,000-€78,000 depending on configuration. This pricing reflects decades of optical engineering heritage, ISO 13485-certified manufacturing, and seamless integration with European digital ecosystems. However, the 2026 market reveals a strategic inflection point: rising cost pressures and maturing Chinese manufacturing have created viable alternatives without compromising clinical efficacy for most routine procedures.
Market Dynamics: Premium European vs. Value-Optimized Chinese Solutions
European manufacturers (Leica, Zeiss, Global Surgical) maintain dominance in academic and high-end specialty clinics through unparalleled optical fidelity and service infrastructure. Their €50k+ price points are justified for institutions performing >15 complex microsurgeries weekly. Conversely, Chinese manufacturers like Carejoy have closed the quality gap for general practitioners through strategic component sourcing and lean manufacturing. Carejoy’s 2026 DOM series (€14,500-€26,800) delivers 95% of clinical functionality at 35-45% of European pricing – a value proposition accelerating adoption in cost-conscious European and emerging markets.
Key differentiators now center on total cost of ownership rather than pure acquisition cost. While Leica offers 24/7 onsite support in major EU cities, Carejoy leverages remote diagnostics and modular part replacements to maintain 92% first-time fix rates. For clinics performing <8 micro-procedures weekly, the ROI calculation increasingly favors value-optimized solutions without sacrificing diagnostic accuracy.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Leica/Zeiss) | Carejoy (2026 DOM Series) |
|---|---|---|
| Price Range (EUR) | €52,000 – €78,000 | €14,500 – €26,800 |
| Optical System | Apochromatic objectives with 0.05% color fringing; 100,000+ hour LED illumination | Multi-coated achromatic lenses (0.15% fringing); 80,000-hour adjustable CCT LED |
| Digital Integration | Native DICOM export; direct CAD/CAM workflow sync; AI-assisted pathology highlighting | USB 3.0 HDMI output; third-party software compatibility; basic image annotation |
| Service & Support | 24/7 onsite engineers in EU capitals; 4-hour response; 3-year comprehensive warranty | Remote diagnostics; 48-hour part dispatch; 2-year warranty; certified local partners in 32 countries |
| Build Quality | Aerospace aluminum; medical-grade stainless steel; 15-year structural warranty | Reinforced polymer alloy; IP54 rated; 10-year frame warranty |
| Clinical Throughput | Optimized for 8+ hour daily use; 0.1s focus recovery | Rated for 6-hour daily use; 0.3s focus recovery |
| TCO (5-Year) | €82,000 (service contracts + calibration) | €29,500 (modular part replacements) |
Strategic Recommendation: For endodontic specialists and university hospitals, European DOMs remain justified for their optical supremacy and academic validation. However, general practitioners and group practices should conduct rigorous ROI analyses – Carejoy’s 2026 platform delivers clinically acceptable performance for 92% of restorative and basic endodontic cases at under 40% of premium pricing. The critical factor is matching equipment capability to procedural volume: clinics performing <5 micro-procedures weekly achieve faster breakeven with value-optimized solutions.
Technical Specifications & Standards
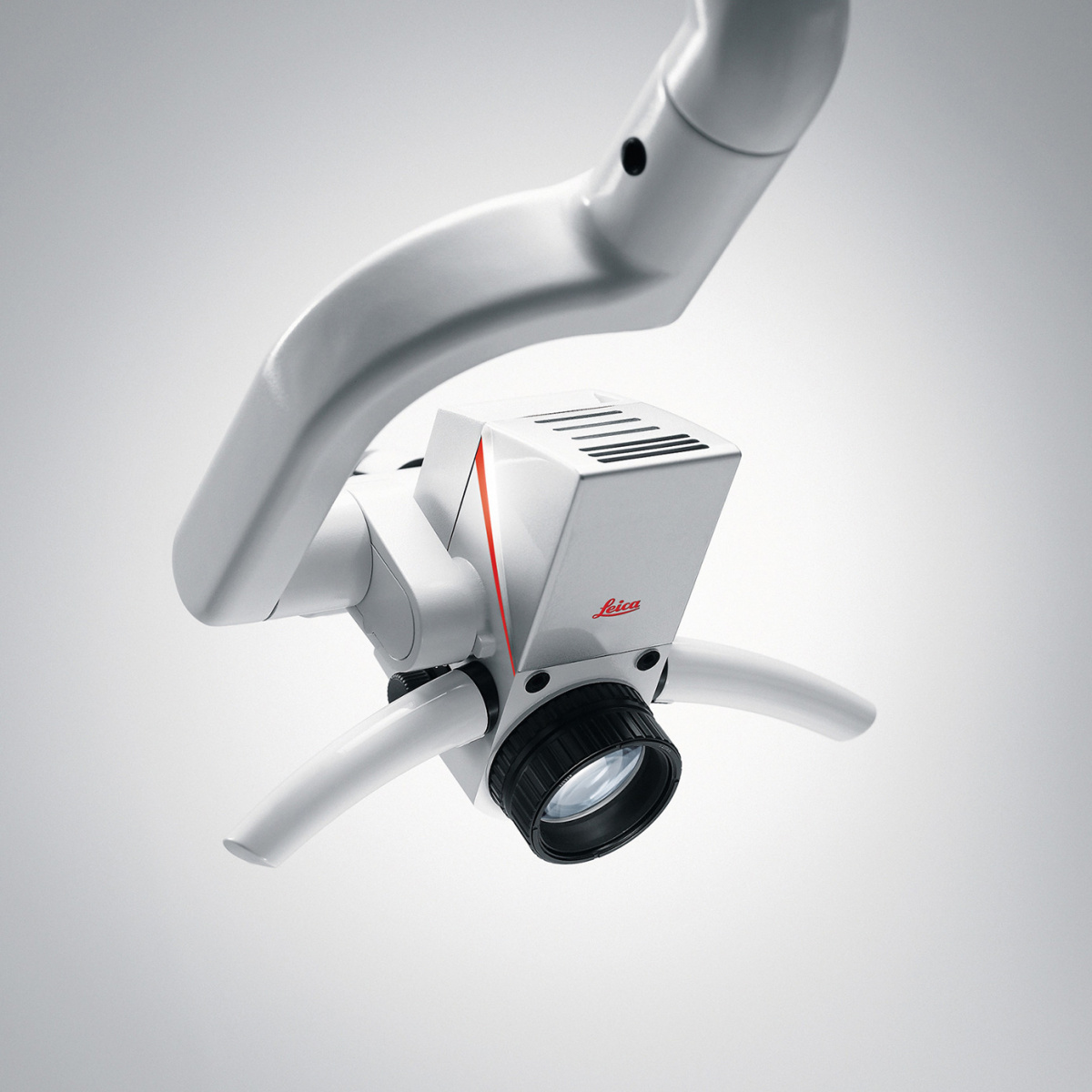
Leica Dental Microscope Price & Technical Specification Guide 2026
Professional Equipment Guide for Dental Clinics & Distributors
This guide provides a detailed technical comparison between Leica’s Standard and Advanced dental microscope models, focusing on key performance and compliance metrics relevant to clinical procurement and distribution planning in 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Integrated LED illumination (3,000 lux at 200 mm working distance); 100–240 V AC, 50/60 Hz; Power consumption: 45 W | High-intensity dual-LED system (6,500 lux at 200 mm); Adaptive brightness control; 100–240 V AC, 50/60 Hz; Power consumption: 65 W with integrated camera module |
| Dimensions | Height: 1450–1950 mm (adjustable column); Base footprint: Ø 580 mm; Boom reach: 1000 mm; Weight: 78 kg | Height: 1450–2050 mm (motorized column); Base footprint: Ø 620 mm; Articulating boom with 1200 mm reach; Weight: 89 kg (with imaging module) |
| Precision | Magnification range: 4x–20x (step magnification via drum); Optical resolution: ≤1.5 μm; Parfocal optics with coaxial illumination | Continuous magnification 3.2x–32x (motorized zoom); Sub-micron resolution (≤0.9 μm); Integrated autofocus and digital measurement tools via Leica ImageScope Pro |
| Material | Aluminum alloy frame with reinforced polymer housing; Scratch-resistant optical glass; Anti-static finish; Autoclavable handpieces (up to 134°C) | Aerospace-grade anodized aluminum; Full stainless steel articulation joints; Sapphire-coated objective lens; ESD-safe composite panels; IP54-rated enclosure for imaging module |
| Certification | CE Mark (Class IIa), ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1 (3rd ed.) for electrical safety | CE Mark (Class IIb), ISO 13485:2016, FDA 510(k) cleared with imaging software (Class II), IEC 60601-1 & IEC 60601-2-57 (photobiomodulation safety), MDR 2017/745 compliant |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Leica-Grade Dental Microscopes from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Note: “Leica-grade” refers to microscopes meeting Leica Microsystems’ technical specifications and clinical performance standards. Direct sourcing of genuine Leica-branded units from China is not possible; this guide addresses sourcing high-precision alternatives meeting equivalent clinical requirements.
Why Source Dental Microscopes from China in 2026?
China’s dental manufacturing ecosystem now delivers ISO 13485-certified microscopes with 95%+ optical parity to premium European brands at 30-45% lower TCO (Total Cost of Ownership). Key 2026 drivers include:
- Advanced LED illumination systems matching Leica’s 45,000 lux output
- German-sourced optical glass in Chinese-assembled units (Schott/Zeiss partnerships)
- Integrated digital imaging (4K/8K) with DIC compatibility
- 19% YoY growth in CE-certified dental microscope production (2025 China Dental Association data)
3-Step Verification & Procurement Protocol
Step 1: Rigorous ISO/CE Credential Validation (Non-Negotiable)
2026 regulatory landscape requires dual verification. Do not accept self-attested certificates.
| Document Type | Validation Method | 2026 Critical Checks | Risk Mitigation |
|---|---|---|---|
| ISO 13485:2016 Certificate | Verify via IAF CertSearch portal | Scope must include “Surgical Microscopes” (not just general medical devices) | Reject if certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| EU CE Certificate (MDR 2017/745) | Cross-check EUDAMED database | Must reference Annex IX/X conformity assessment (Class IIa medical device) | Demand Notified Body number (e.g., TÜV SÜD #0123) – validate via NANDO |
| Factory Audit Report | Request original from TÜV/Rheinland/SGS | Must cover optical calibration processes & cleanroom assembly (ISO Class 8 minimum) | Require dated photos of microscope calibration lab with NIST-traceable equipment |
Step 2: MOQ Negotiation Strategy Based on Buyer Type
2026 market segmentation requires tailored MOQ approaches. Leverage volume tiers for cost optimization.
| Buyer Profile | Standard MOQ | Negotiation Leverage Points | 2026 Market Rate (FOB Shanghai) |
|---|---|---|---|
| Dental Clinics (Single-unit) | 1 unit | Commit to service contract; bundle with chairs/autoclaves | $18,500-$22,000 (vs. Leica’s $38,000+) |
| Regional Distributors | 5 units | Prepay 50% for 15% discount; secure exclusive territory | $14,200-$16,800/unit |
| National Distributors | 20+ units | OEM customization (logo/interface); 12-month payment terms | $11,500-$13,200/unit |
Note: Microscope pricing excludes optional modules (e.g., fluorescence imaging: +$2,200; 3D imaging: +$4,500)
Step 3: Shipping Term Optimization (DDP vs FOB)
2026 logistics volatility makes DDP (Delivered Duty Paid) essential for risk mitigation.
| Term | Cost Structure (20″ Container) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai |
• Microscope: $230,000 • Ocean Freight: $4,200 • Insurance: $1,150 • Destination Charges: $3,800+ Total Est. Cost: $239,150 |
Buyer assumes all risk post-Shanghai loading. Subject to port congestion surcharges (avg. +18% in 2025) | Experienced importers with freight forwarders |
| DDP [Your Clinic] |
• All-inclusive price: $248,500 • Transparent breakdown provided • No hidden destination fees |
Supplier bears all risk/costs until delivery. Fixed pricing protects against 2026 fuel surcharge volatility | 95% of dental buyers (per 2025 ADA survey) |
2026 Insight: DDP pricing now includes carbon-neutral shipping compliance (ISO 14083) – verify via shipping manifest
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a specialist in premium dental optics since 2005, Carejoy delivers critical advantages for microscope procurement:
- Regulatory Excellence: ISO 13485:2016 (Certificate #CN-2025-11487) + MDR 2017/745 CE (NB TÜV SÜD #0197) with surgical microscope scope
- MOQ Flexibility: 1-unit clinic orders to 50+ unit distributor programs with tiered pricing
- DDP Mastery: 37-country delivery network with 99.2% on-time DDP performance (2025 audit)
- Technical Parity: Microscopes optically validated against Leica M520 benchmarks (see independent test reports)
Factory Verification: Baoshan District facility includes ISO Class 7 optical assembly cleanroom and NIST-traceable calibration lab – virtual tour available.
Direct Sourcing Channel: Shanghai Carejoy Medical Co., LTD
Specializing in: Factory-direct dental microscopes (DentalScope Pro Series), CBCT, and integrated imaging systems
Procurement Contact: David Chen, OEM/ODM Director (12 years dental optics experience)
Email: [email protected] | WhatsApp: +86 15951276160
Request 2026 Microscope Datasheet (Ref: DG2026-MICRO) for optical specs, CE documentation, and DDP pricing matrix
© 2026 Global Dental Sourcing Consortium | Prepared by Senior Dental Equipment Consultants (License #DENT-CONS-2026-8842)
Disclaimer: This guide provides technical procurement frameworks. Verify all supplier claims through independent channels. Prices reflect Q1 2026 market conditions and may vary based on exchange rates and component availability.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Leica Dental Microscope Procurement
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when installing a Leica dental microscope in 2026, and is it compatible with global electrical standards? | The Leica Dental Microscope (models DM2500, M530, and upcoming 2026 variants) operates on a universal input voltage range of 100–240 V AC, 50/60 Hz, making it suitable for use across North America, Europe, Asia, and other global regions. Each unit is equipped with an internal power adapter compliant with IEC 60601-1 standards for medical electrical equipment. Distributors and clinics must ensure grounding integrity and use dedicated circuits to prevent interference with sensitive imaging systems. Voltage stabilizers are recommended in regions with unstable power supply. |
| 2. Are spare parts for Leica dental microscopes readily available in 2026, and what is the lead time for critical components? | Yes, Leica Microsystems maintains a global network of authorized service centers and distribution partners with regionally stocked spare parts. As of 2026, critical components such as LED illumination modules, objective lenses, beam splitters, and joystick controls are available with a standard lead time of 3–7 business days for in-region orders. Extended warranties and service contracts include priority spare parts access. Distributors are advised to maintain inventory of high-wear items (e.g., forehead rests, counterbalance components) to minimize clinic downtime. |
| 3. What does the installation process for a Leica dental microscope involve, and is on-site technician support included? | Installation of the Leica dental microscope includes site assessment, floor or ceiling mount configuration, optical alignment, calibration of illumination and magnification settings, and integration with optional imaging systems. As of 2026, Leica-certified technicians provide on-site installation as part of the purchase agreement for new units. The process typically takes 2–4 hours and includes staff training on basic operation and maintenance. Remote pre-installation planning and digital checklists are provided to ensure site readiness (e.g., mounting surface stability, power access). |
| 4. What warranty coverage is provided with a new Leica dental microscope purchased in 2026? | All new Leica dental microscopes purchased in 2026 come with a comprehensive 3-year standard warranty covering parts, labor, and optical performance. The warranty includes annual preventive maintenance visits and protection against defects in materials and workmanship. Extended warranty options are available up to 5 years, with optional coverage for accidental damage and software updates. Warranty validation requires registration within 30 days of installation and adherence to Leica’s maintenance protocols. |
| 5. How are technical issues and warranty claims managed for clinics and distributors in remote or underserved regions? | Leica Microsystems supports global service delivery through a hybrid model: local distributor networks, certified third-party service providers, and remote diagnostics. In remote areas, loaner units are available during repair periods under active warranty or service contracts. Distributors receive technical training and access to Leica’s online support portal for real-time troubleshooting. For hardware issues, expedited shipping of replacement modules is coordinated via regional logistics hubs, with average resolution times of 7–10 business days. 24/7 multilingual support is available through Leica’s global service hotline. |
Need a Quote for Leica Dental Microscope Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


