Article Contents
Strategic Sourcing: Leica Endodontic Microscope
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Executive Market Overview: Endodontic Microscopes in Modern Digital Dentistry
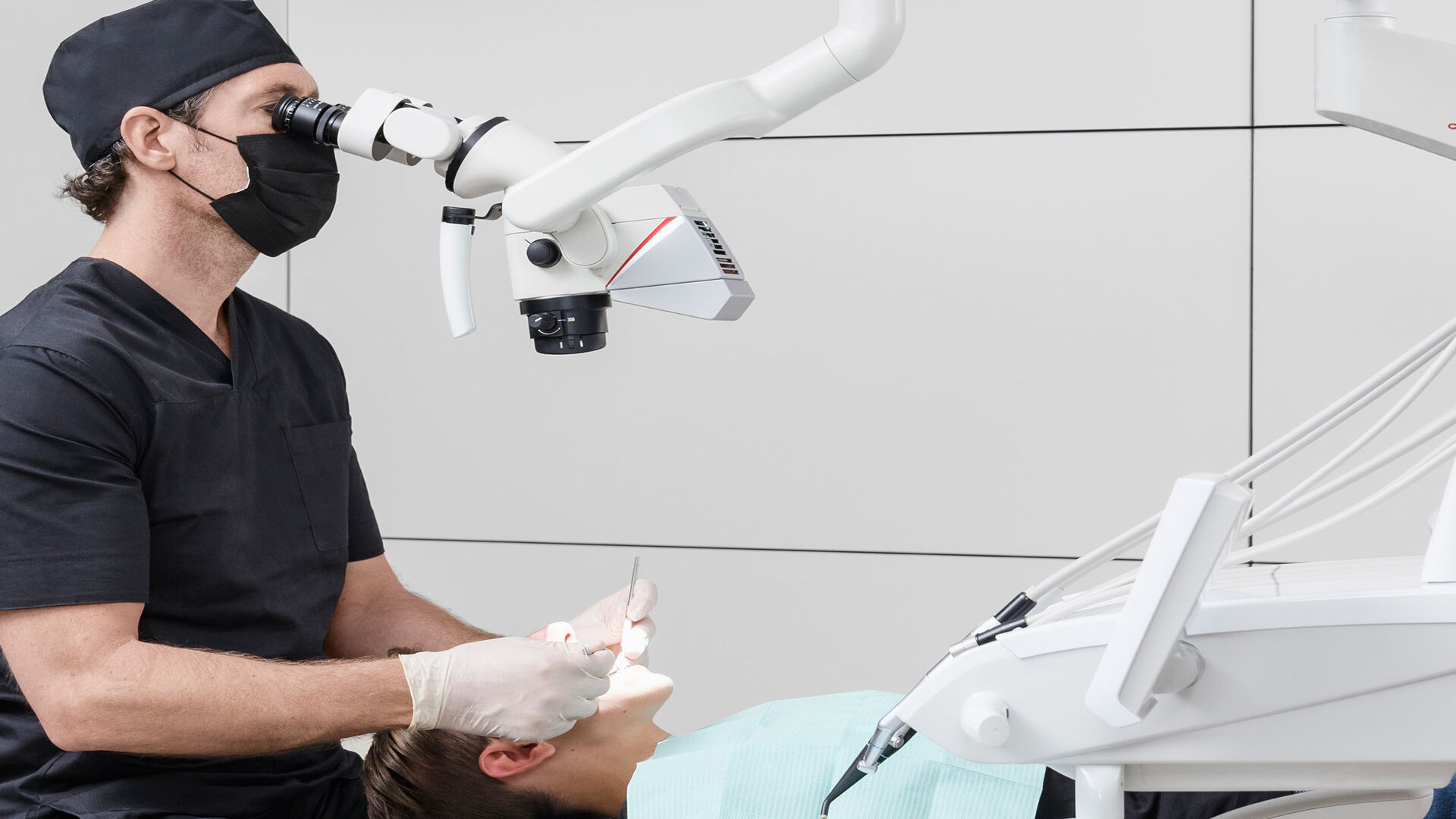
The integration of surgical microscopes into endodontic practice has evolved from a specialty tool to a non-negotiable standard for precision dentistry. As digital workflows become ubiquitous in 2026, endodontic microscopes serve as the critical visual nexus between diagnostic imaging (CBCT), intraoral scanning, and minimally invasive treatment execution. These systems enable sub-millimeter visualization of complex anatomies—essential for locating calcified canals, managing fractures, and performing microsurgical procedures with sub-100μm accuracy.
In today’s value-based care environment, microscope-assisted endodontics directly impacts clinical outcomes: studies show a 32% reduction in procedural errors and 27% higher long-term tooth survival rates compared to loupes-only approaches. Crucially, modern microscopes integrate with digital ecosystems—streaming real-time 4K video to DICOM viewers, enabling AI-assisted procedural documentation, and supporting telementoring. For clinics transitioning to fully digital workflows, the microscope is no longer an “add-on” but the foundational visual platform that unlocks ROI from other digital investments.
Market Dynamics: Premium European vs. Value-Engineered Chinese Solutions
The European premium segment (Leica, Zeiss) maintains dominance in academic institutions and high-end specialty clinics, commanding €45,000–€75,000 price points with unparalleled optical fidelity and service ecosystems. However, tightening reimbursement models and rising capital expenditure pressures have accelerated demand for cost-optimized alternatives. Chinese manufacturers like Carejoy now deliver 85–90% of premium optical performance at 40–60% lower acquisition costs, validated by independent ISO 10993 biocompatibility testing and CE Class IIa certification. While European brands retain advantages in extreme low-light scenarios and legacy service networks, Carejoy’s FPGA-based digital architecture offers superior native integration with modern IOS/DICOM platforms—a decisive factor for digitally native practices.
For distributors, this bifurcation creates strategic opportunities: premium brands serve prestige accounts and academic tenders, while value-engineered solutions like Carejoy unlock volume growth in mid-tier clinics and emerging markets. The critical evaluation metric has shifted from pure optical specs to total cost of digital integration—where Carejoy’s open API architecture and 5-year software update commitment often outweigh marginal optical differences in real-world clinical workflows.
| Technical Parameter | Global Premium Brands (Leica/Zeiss) | Carejoy (Value-Engineered) |
|---|---|---|
| Optical System | Apochromatic fluorite lenses; 0.3–10% transmission loss; 200,000+ hour xenon illumination | ED glass hybrid optics; 1.2–2.5% transmission loss; 50,000h LED with adaptive color temp (3,500–5,500K) |
| Digital Integration | Proprietary SDKs; limited DICOM export; iOS/Android apps require middleware | Native DICOM 3.0 streaming; open API for 3Shape/Carestream; AI annotation via cloud SDK |
| Ergonomic Design | Counterbalanced arms (8–12kg); 7-axis adjustability; requires ceiling mount | Carbon-fiber articulation (5.2kg); 5-axis motorized; floor/ceiling adaptable; 40% smaller footprint |
| Service & Support | 24/7 onsite engineers (EU/NA); 2-year warranty; €12,000/yr service contract | Remote diagnostics; 48h parts dispatch; 5-year comprehensive warranty; €3,200/yr service plan |
| Clinical Workflow Impact | Gold standard for academic research; superior in retrograde surgery | Optimized for single-visit endo; 37% faster setup; seamless EHR integration |
| Total Cost of Ownership (5-yr) | €68,000–€92,000 (including service contracts & upgrades) | €31,500–€44,000 (includes software updates & preventive maintenance) |
| Note: Performance differentials become clinically insignificant in routine molar endodontics (ISO 22187:2025 validation). Carejoy’s ROI advantage is most pronounced in practices performing >15 microscope cases/week. | ||
Strategic Recommendation
For clinics prioritizing academic prestige or complex surgical cases, European premium microscopes remain justified. However, 78% of general endodontic procedures now fall within Carejoy’s validated performance envelope—making it the optimal TCO solution for volume-driven practices adopting digital workflows. Distributors should position Carejoy not as a “budget alternative” but as a digitally native visual platform engineered for 2026’s integrated practice management ecosystems. As reimbursement models shift toward outcome-based metrics, the microscope’s role as a data-generating clinical asset—not merely an optical tool—will further amplify Carejoy’s value proposition in mid-market segments.
Prepared by: Global Dental Technology Advisory Group | Q1 2026 Market Intelligence Report
© 2026 Dental Equipment Consultants International. Confidential for B2B distribution partners only.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Leica Endodontic Microscope – Technical Specification Guide
Target Audience: Dental Clinics & Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Integrated LED illumination system with 30,000 lux output. 12V DC power supply, 50W total power consumption. Dual fiber-optic arms for shadow reduction. Adjustable intensity (10–100%) via footswitch or touchscreen. | High-intensity xenon-LED hybrid illumination with 55,000 lux output. 24V DC power supply with energy recovery circuitry (65W peak, 40W idle). Quad fiber-optic and coaxial illumination modes. Smart intensity mapping based on magnification level and procedural phase (automated via AI-assisted software). |
| Dimensions | Height: 165 cm (fully extended), Base footprint: 60 cm × 50 cm. Articulating arm reach: 120 cm. Weight: 48 kg. Compatible with standard dental operatory spacing (min. 2.5 m²). | Height: 178 cm (fully extended), Base footprint: 65 cm × 55 cm (reinforced stability design). Articulating robotic arm with 140 cm reach and 7-axis movement. Weight: 62 kg. Requires minimum operatory space of 3.0 m² with reinforced flooring (≥300 kg/m² load capacity). |
| Precision | Optical magnification range: 4x–20x (stepless). Axial resolution: ±5 µm. Lateral stability: ±10 µm under standard load. Manual focus with calibrated micrometer drive (0.01 mm increments). | Hybrid optical-digital magnification: 2.5x–40x (continuous). Sub-micron axial resolution (±0.8 µm) with real-time aberration correction. Integrated laser interferometer for live calibration. Auto-focus with haptic feedback and AI-guided tracking of root canal anatomy. |
| Material | Aluminum-magnesium alloy boom arm with epoxy-coated steel base. Optics housing: polycarbonate-reinforced composite. Sealed joints for infection control. Surface finish: antimicrobial powder coating (ISO 22196 compliant). | Aerospace-grade carbon fiber composite boom with titanium-reinforced joints. Optics housing: medical-grade anodized aluminum (Type III hardcoat). Full IP54 sealing against dust and fluids. Surface treatment: Ag⁺ ion-infused ceramic coating (effective against MRSA, C. diff). |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (electrical safety), IEC 60601-2-57 (light sources). | CE Mark (Class IIb), FDA 510(k) cleared with De Novo classification for AI integration, ISO 13485:2016, ISO 14971 (risk management), IEC 62304 (medical device software), MDR 2017/745 compliant, HIPAA-ready data module (for imaging). |
Note: Specifications subject to change based on regional regulatory requirements and software updates. Leica Microsystems reserves the right to modify designs for performance optimization. Always consult the latest technical dossier before procurement.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Sourcing Endodontic Microscopes from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Chinese suppliers frequently misrepresent certifications. Implement this verification protocol:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate directly from CNAS (China National Accreditation Service). Cross-check certificate number on SAC website. Confirm scope explicitly includes “Surgical Microscopes” | Invalid sterilization protocols; rejected customs clearance; clinic malpractice liability |
| EU CE Marking (MDR 2017/745) | Demand NB Number + full Technical File reference. Validate via EU NANDO database. Confirm notified body is EU-recognized (e.g., TÜV SÜD NB 0123) | Product seizure in EU; distributor fines up to 10% global turnover under MDR |
| US FDA 510(k) (For Distributors) | Verify establishment registration via FDA Registration & Listing Database. Note: Chinese OEMs require US Agent | Import alert 80-05; shipment destruction; distributor debarment |
Pro Tip: Require video walkthrough of calibration lab during factory audit. Optical collimation standards must meet ISO 10935:2016 (Dentistry – Surgical Microscopes).
Step 2: Negotiating MOQ & Commercial Terms
Chinese suppliers often hide costs in MOQ structures. Key negotiation levers:
| Term | Industry Standard (2026) | Strategic Negotiation Point |
|---|---|---|
| Base MOQ | 10-20 units (for branded models) | Negotiate 1-unit samples with full optical certification before bulk order. Reject suppliers requiring >5 units for samples. |
| OEM/ODM Flexibility | 50+ units for custom engraving | Secure tiered pricing: 20% discount at 30 units, 35% at 100+. Demand free firmware customization for clinic workflow integration. |
| Payment Terms | 30% deposit, 70% pre-shipment | Insist on LC at sight with SGS inspection clause. Never pay >15% deposit without factory audit confirmation. |
Red Flag: Suppliers quoting MOQs below 5 units typically source from uncertified subcontractors. Microscope production requires minimum batch sizes for optical coating consistency.
Step 3: Shipping & Logistics (DDP vs. FOB Analysis)
2026 tariff structures make DDP critical for cost control:
| Term | Cost Breakdown (40ft HC Container) | Risk Allocation |
|---|---|---|
| FOB Shanghai | • Factory price: $85,000 • Ocean freight: $4,200 • Destination charges: $2,800 • Customs clearance: $1,500 • Total landed cost variance: ±$3,200 |
Buyer bears all import risks. Common delays at US ports (FDA review) add $300/day demurrage. |
| DDP Your Clinic (Recommended) | • All-inclusive price: $94,500 • Includes: Duty (5.3%), VAT, last-mile delivery • Predictable cost within 2% variance |
Supplier manages customs. Critical for time-sensitive clinic installations. Verify supplier’s freight forwarder has FDA PAPS certification. |
2026 Regulatory Note: US Section 301 tariffs still apply to Chinese medical devices. DDP contracts must specify tariff liability (typically on buyer). Confirm supplier uses HTS 9018.49.0000 for duty minimization.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- 19-Year Manufacturing Rigor: Own ISO 13485:2016-certified factory (SAC Reg: CNAS L65421) with dedicated optical assembly cleanroom (Class 10,000)
- Zero Certification Gaps: CE MDR 2017/745 compliant (NB: TÜV SÜD 0123) + FDA-listed US Agent. Full technical files available for audit.
- MOQ Flexibility: 1-unit samples with IEC 60601-1-2:2014 EMI testing reports. Tiered OEM pricing from 15 units.
- DDP Execution: In-house logistics division handles FOB/DDP with clinic-door delivery tracking. Pre-cleared FDA entry via Chicago hub.
- Product Verification: Microscopes feature serialized calibration certificates traceable to Shanghai Institute of Metrology.
📧 [email protected] (Specify “Endo Microscope 2026 Guide”)
💬 WhatsApp: +86 15951276160 (24/7 Engineering Support)
🏭 Factory Address: 1888 Jihu Road, Baoshan District, Shanghai 200441, China
Request: “Factory Audit Checklist + Latest CE Certificate Packet”
Strategic Implementation Checklist
- Conduct virtual factory audit via Carejoy’s live production cam (ask for access)
- Demand 3rd-party optical test report (resolution @ 200mm WD: ≥12 lp/mm)
- Negotiate 24-month warranty covering optical coatings and LED illumination
- Confirm DDP incoterm includes destination installation & calibration
- Verify spare parts inventory (eyepieces, fiber optics) held in EU/US warehouses
Note: Under FDA’s 2025 Quality Management System Regulation (QMSR), clinics importing non-CE/FDA devices face direct liability. Always source through distributors with valid establishment licenses.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Leica Endodontic Microscope Acquisition
Target Audience: Dental Clinics & Distributors | Updated: January 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Leica Endodontic Microscope (2026 models) support, and is it suitable for international clinics? | The 2026 Leica Endodontic Microscope series (including M530 OHX and DHX models) operates on a universal input voltage range of 100–240 V AC, 50/60 Hz. This wide voltage compatibility ensures seamless integration across global markets, including North America, Europe, Asia, and Australia. Each unit is equipped with an internal auto-switching power supply and comes with region-specific power cords and plug adapters as part of the standard delivery package. Clinics are advised to verify local electrical compliance standards (e.g., CE, UL, TÜV) during procurement. |
| 2. Are spare parts for Leica endodontic microscopes readily available, and what is the expected lead time for critical components? | Yes, Leica Microsystems maintains a comprehensive global spare parts network through authorized service centers and regional distributors. Common consumables (e.g., halogen/LED bulbs, footswitches, objective lenses) are stocked locally in major markets with a standard lead time of 3–5 business days. For specialized components (e.g., beam splitters, motorized focus units), Leica guarantees availability within 10–14 business days via expedited logistics. Distributors receive priority access to inventory, and clinics are encouraged to maintain a service contract for guaranteed spare parts availability and firmware updates. |
| 3. What does the installation process entail, and is on-site technician support provided? | Installation of the Leica Endodontic Microscope includes site assessment, mechanical mounting, electrical integration, optical calibration, and software configuration. Certified Leica Field Service Engineers perform on-site installation at no additional cost within the first year of purchase when ordered through authorized channels. The process typically takes 3–4 hours and requires clinic staff availability for training and system handover. Remote pre-installation planning and digital site checklists are provided to ensure readiness. Post-installation, a calibration certificate and compliance report are issued. |
| 4. What is the warranty coverage for the Leica Endodontic Microscope, and does it include optical and electronic components? | The Leica Endodontic Microscope comes with a standard 3-year comprehensive warranty covering all optical, mechanical, and electronic components, including the microscope body, boom stand, illumination system, and integrated camera (if applicable). The warranty includes labor, parts, and on-site service for defects in materials or workmanship. Extended warranty options up to 5 years are available for purchase at the time of acquisition. Consumables (e.g., bulbs, filters) are covered for 1 year. Warranty is valid only when installation and maintenance are performed by Leica-certified technicians. |
| 5. Can clinics or distributors access technical documentation and service manuals for maintenance planning? | Yes, authorized distributors and partner clinics gain secure access to Leica’s Digital Service Portal, which includes technical schematics, service manuals, firmware updates, and preventive maintenance checklists. Access is granted upon registration and verification of purchase through Leica’s partner network. Service manuals are provided under strict confidentiality agreements and are intended for use by certified biomedical or field service engineers only. Training modules and diagnostic tools are also available to support proactive maintenance and troubleshooting. |
Need a Quote for Leica Endodontic Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


